
Encouraging pharmaceutical innovation in middle-income countries
By Tim Wilsdon, Vice President, and Eva Fiz, Consulting Associate, at Charles River Associates
With each newspaper report on new investments in China or Brazil (and research center closures in Europe), the changing landscape of pharmaceutical research and development (R&D) is becoming ever more evident. However, the factors driving these changes, the importance of intellectual property (IP) and the implications for government policy remain subjects of contention and debate. To better understand the dynamics of innovation within the pharmaceutical industry, the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) asked economic consultancy firm Charles River Associates (CRA) to analyze the conditions that enable pharmaceutical innovation to thrive and the potential future implications for innovation policies, with a specific focus on a selection of key middle-income countries.
Drawing on interviews with policymakers, international and domestic companies, and academics, CRA assessed the innovative activities in middle-income countries and the degree to which these activities can be associated with public policy in a range of case study countries (Brazil, Colombia, China, India, Malaysia, South Africa, the Republic of Korea and the Russian Federation).
Although there is increasing innovative activity in all countries considered, the opportunity to develop innovative activities from basic research through to clinical development varies from country to country. To be successful, a jigsaw of policies is needed, including a coordinated industrial and health policy, strong IP protection and an environment that encourages partnerships among the different stakeholders.
Recent trends

Location of innovative activities at each stage of the R&D process. Source: CRA analysis. The
location of R&D hubs is based on public information of IFPMA members as of August 2012. The
number of clinical trials is based on www.clinicaltrials.gov.
To understand recent trends and policy challenges, it is important to differentiate between types of biopharmaceutical innovation. Innovative activity is typically divided into basic research (sometimes described as drug discovery), preclinical research and clinical trials (which themselves are divided into Phases I to III (registration), and Phase IV (post-registration) trials).
Biopharmaceutical innovative activities are primarily concentrated in high-income countries; however, there is a clear growth trend in these activities in middle-income countries. Between 2005 and 2010, industry R&D spending increased by 455 percent in Asia-Pacific (excluding Japan), 112 percent in Latin America, and 303 percent in India.
Early-stage research is undertaken by international pharmaceutical companies working closely with leading academic centers in research hubs. Historically, these have focused on regions such as Boston and San Francisco in the US and London and Cambridge (UK), Uppsala (Sweden) and Munich (Germany) in Europe, and Singapore in Asia. Among middle-income countries, however, China stands out as the home of 12 R&D centers. A few R&D hubs are also established in India, Brazil, the Russian Federation and Indonesia.
The trend towards biopharmaceutical innovative activities in middle-income countries is even clearer when looking at later stages of the R&D process. For example, clinical research is undertaken in many locations with middle-income countries now hosting 15 percent of global clinical trial activity. China, India, the Russian Federation and Brazil have captured the largest number of trials within those markets.
Ultimately, the success of the innovation strategies of middle-income countries should be judged on their outputs. Although it is difficult to tie medicines developed by the international industry to a particular market, there is evidence of incremental innovation, where medicines are tailored to the local markets in middle-income countries. Furthermore, there are a significant number of innovative products in Phases II and III, and a number of commercialized novel medicines have been developed indigenously in some case study countries, such as the Republic of Korea, China and India. However, at the present time there are no international blockbuster drugs emerging from the case study countries.
The role of IP in determining R&D location
The factors determining the location of innovation are complex and difficult to disentangle. It is clear that to develop innovative activity (particularly early-stage research) governments must have a coherent long-term policy that is implemented effectively.
The biopharmaceutical process of innovation is long, often taking 10 to 12 years to progress from proof of concept to global commercialization. As investment in early-stage research is not a linear process, it is difficult to assign clear time frames or costs to the patented outputs that result. Given the unpredictable timing and the significant costs associated with establishing new research centers, it is unsurprising that decisions regarding location are rare but, when they do occur, they are strategically important. In future, decisions on new investments are likely to be even more difficult, given the ongoing trend to consolidate R&D sites. However, as illustrated above, although the great majority of international R&D sites continue to be in the US and Europe, the importance of China as an R&D hub has increased dramatically.
This is, in part, due to China's long-term commitment to promoting innovation in the pharmaceutical industry, which is seen as an important signal that the future innovation environment in China promises to be favorable. China has applied coherent plans to encourage both public and private biopharmaceutical innovation since 2006.
The requirements for developing strong innovative capacity differ depending on whether consideration is given to early-stage research or later-stage development. Early-stage and preclinical research requires the best academic and research capabilities. All companies interviewed agreed that the availability of “talent” was the central justification in considering the location of their early-stage facilities. Although talent can be recruited to a location, a world-class institution or research group was seen as the essential building block in developing this capability. Moreover, to be effective, academia needs to create a culture of collaboration with private companies, which would inevitably be involved in the development of the medicines. Such a culture takes time to develop and is lacking in the majority of the countries included in the analysis.
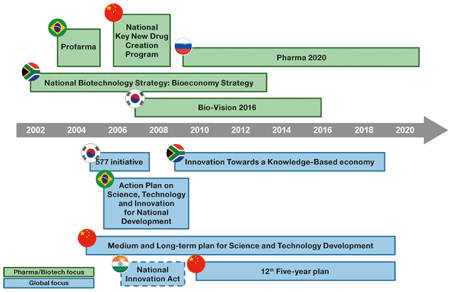
National innovation strategies. (Source: CRA analysis. *The Indian National Innovation Plan was
drafted in 2008 but not implemented. The new innovation plan currently being drafted, the
Science and Technology Innovation Policy, is expected to be announced in 2013.
**Amendments to the program were proposed due to budget concerns.)
Clinical development relies on considerable expertise and experience in managing and supervising trials according to international standards. The motivation for undertaking these trials increases greatly when a product is destined for the domestic market, and local evidence and clinical support is required for its appraisal. Not all countries have the market size or even the population to encourage large scale clinical trials. A staged, targeted, consistent and coordinated policy framework is therefore required to build capabilities to undertake the large-scale trials needed for late-stage clinical development.
The prevailing innovation model depends on patents as a basis for securing a return for all those who invest in different ways at different stages of the innovation process. In light of this, decisions about where to locate basic and preclinical research facilities depend, to a large extent, on the IP regime of the country. International and domestic companies will only invest in the risky research process if it is possible to protect the IP associated with their investments. Based on the interviews, policymakers reported that confidence in their IP regime was crucial, and robust IP rules could represent a key competitive advantage in encouraging domestic innovative activity.
The study found that international companies would only invest in research in locations with sufficiently strong IP protection. This is one of the reasons why China has been relatively more successful in attracting inward investment in research. For domestic pharmaceutical innovators who are more heavily reliant on rewards from the domestic marketplace, the importance of IP is even greater.
To a lesser extent, decisions regarding the location of clinical trials depend on the protection available during the trial process. Overall, IP protection plays a role in prioritizing clinical trial sites, because all things being equal, companies would rather conduct trials in countries where their patent rights will be upheld.
The interviews revealed that if the objective is to develop an innovative biopharmaceutical industry (either by fostering domestic companies or attracting investment by international companies), IP is a necessary (although not sufficient) building block.
Partnership is key to early-stage research
The case studies also illustrate that once the basic infrastructure is developed, public investment in R&D is not enough to ensure a sustainable innovative industry. Partnership is vital for encouraging early-stage research. For clinical research, partnerships appear to develop with less government involvement. Market forces, in particular, seem to be primarily responsible for the development of domestic clinical research organizations (CROs) and their success in attracting clinical trials.
The global pharmaceutical industry has played a significant role in fostering innovation in emerging markets over the last 10 years. This is perhaps partly in response to the slower growth in its core business in western markets. The industry has adopted a strong, positive approach to engaging with middle-income markets (particularly China). Not only has it been investing in local marketing affiliates, but also in partnering with the emerging university and government research institute fraternity and, in a number of cases, with established independent corporate research centers.
Summary of conclusions
- Policies need to be tailored to the market size and population of each country;
- Building capabilities over time in the areas in which a country can compete internationally requires considerable time and investment and involves the development and step-wise application of targeted programs;
- Fostering innovation throughout the drug development pipeline from basic research through to clinical development requires a jigsaw of policies including a consistent policy framework, a coordinated industrial and health policy, strong IP protection and an environment that encourages partnerships among stakeholders.
Further research needed
Although there is a vast literature on the determinants of innovation and innovation policy in general, there are still considerable gaps in the current evidence and research. For example, further study is required to better understand the factors that determine different types of foreign direct investment and how these relate to the characteristics of a given IP regime.
The WIPO Magazine is intended to help broaden public understanding of intellectual property and of WIPO’s work, and is not an official document of WIPO. The designations employed and the presentation of material throughout this publication do not imply the expression of any opinion whatsoever on the part of WIPO concerning the legal status of any country, territory or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication is not intended to reflect the views of the Member States or the WIPO Secretariat. The mention of specific companies or products of manufacturers does not imply that they are endorsed or recommended by WIPO in preference to others of a similar nature that are not mentioned.