Chapter 6 India
Authors: Justice Madan B. Lokur (ret.), Justice Gautam Patel, Justice Prathiba M. Singh and Justice Manmohan Singh (ret.)
6.1 Overview of the patent system
6.1.1 Evolution of the patent system
Intellectual property (IP) rights are governed by national law, which for members of the World Trade Organization (WTO), shall be in conformity with the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement).1 The TRIPS Agreement sets out the objective of IP rights in Article 7:
The protection and enforcement of intellectual property rights should contribute to the promotion of technological innovation and to the transfer and dissemination of technology, to the mutual advantage of producers and users of technological knowledge and in a manner conducive to social and economic welfare, and to a balance of rights and obligations.
As a member-nation of the WTO, India was required to amend or enact laws to conform to the TRIPS Agreement. However, this was a challenge for India. A significant reason was that, unlike many other countries, such as the United States of America (U.S.), where the Constitution recognizes the promotion and progress of science and arts and secures exclusivity granted to authors and inventors, the Constitution of India only encourages Indian citizens to have a scientific temper and prescribes a duty to develop the spirit of inquiry and reform.2 The Constitution of India mandates that no one shall be deprived of “property” except with the authority of law.3 Since patents are “property,” there is a positive constitutional entitlement to the grant and recognition of patents. The non-enforceable – but critical – chapter of the “Directive Principles of State Policy” in the Constitution of India further directs the Government to ensure the promotion of public health,4 the reduction of inequalities5 and the securing of systems that ensure ownership and control of resources for the common good.6 The basis and limitations for IP rights are, therefore, the right to property, the directive principles of state policy and the fundamental duties of citizens, apart from the various laws enacted periodically.
The journey of the Indian patent regime is reflected in three different periods: colonization, post-independence and globalization.7
Colonization. India inherited its patent regime from the British rule. When the British colonization of India ended, the Indian Patents and Designs Act, 1911, was in force and had created a system of patent administration in India under an administrative office – the Controller of Patents and Designs.
Post-independence. India enacted its first independent patent law in 1970. It came in the backdrop of two committees constituted to make recommendations: the Bakshi Tekchand Committee in 1949 and, later, the Justice Rajagopal Ayyangar Committee. Focusing on the special socioeconomic conditions in India, the recommendations of these two committees resulted in far-reaching changes in patent laws. Some of the significant changes introduced were with respect to food and drug patents, compulsory licensing, and connected working requirements. The law enacted in 1970 is credited with the growth of various industries, including the pharmaceutical industry, which, in two decades, gave India the distinction of being called “the pharmacy of the world” as Indian drug companies began exporting reasonably priced medicines to many countries.
Globalization. In 1991, India liberalized its economy and adhered to the General Agreement on Tariffs and Trade (GATT 1947), which was succeeded by the WTO, resulting in amendments being introduced in line with the TRIPS Agreement. These amendments saw India bring about fundamental changes permitting product patents in food, medicines and agrochemicals. The flexibilities in the TRIPS Agreement were used to maintain a balance: ensuring that the amendments would be gradually made systemic rather than forcing the closure of already-functioning industries. Statutory provisions relating to chemical and drug patents, patentability and other aspects of the amendments were tested repeatedly in the courts and were upheld as being within the Constitutional scheme while being fully compliant with the TRIPS Agreement. The judgment of the Supreme Court in Novartis v. Union of India8 recognized the need to curb the “evergreening” of patents while acknowledging the need to grant patent protection to incremental innovations. After Novartis, Indian courts have granted interim injunctions to protect patentees’ rights in pharmaceutical9 and agrochemical inventions.10 The courts have also protected claims to standard-essential patents (SEPs) by granting interim injunctions to secure the patentee’s right to royalties even pending trial.11 Courts have granted permanent injunctions12 and damages (in quite significant amounts)13 in cases of patent infringement and have also denied interim injunctions in appropriate cases.14 Each case has been decided on its own facts on the basis of settled legal principles. A current review of decisions would show no pro- or anti-patentee bias in the adjudication of patent cases.
6.1.2 The Justice N Rajagopala Ayyangar Committee Report
In 1957, the Government of India appointed a committee led by a distinguished retired Justice of the Supreme Court of India, Justice N Rajagopala Ayyangar, to examine the revision of the Patents Act and advise the Government in this respect.
The Justice N Rajagopala Ayyangar Committee report stated, in no uncertain terms, that the patent system was a quid pro quo system: the monopoly that a patentee obtains is only in exchange for the disclosure of the invention to the public, free to be used after the monopoly period is over. The quid pro quo, according to the report, also included the obligation on the part of the patentee to work the invention in India. The report also underscored, rather emphatically, that the patent system had failed in India because it had failed to spark the kind of innovation that it sought to encourage – underdeveloped countries could not yield the same result from the patent system as their more developed counterparts could. The patent system was recommended to be continued only because there was no better alternative to achieve better results – in their form at the time, patents were the lesser evil. The report was unequivocal in its apprehension that foreign patentees could misuse the patent system to capture large markets in India at the cost of domestic innovation while simultaneously not investing in the manufacture of the patented product.
The committee’s recommendations were a catalyst for wide changes in Indian patent law, eventually leading to the Patents Act of 1970, replacing the Indian Patents and Designs Act, 1911. The Patents Bill was introduced in 1965 and amended in 1967. The Patents Act, 1970, and Patents Rules, 1972 came into force on April 20, 1972.
6.1.3 The Patents Act, 1970 (pre–TRIPS Agreement)
The Patents Act, 1970, incorporated major provisions to reduce the social costs of foreign-owned patents. It prohibited patents on products useful as medicines and food, shortened the term of chemical process patents, and significantly expanded the availability of compulsory licensing. This spawned a powerful Indian pharmaceutical generic drugs industry.
In Bishwanath Prasad Radhey Shyam v. HM Industries,15 deciding an appeal in a case for infringement of a patent called “Means for Holding Utensils for Turning Purposes,” the Supreme Court said:
The object of the patent law is to encourage scientific research, new technology and industrial progress. Grant of exclusive privilege to own, use or sell the method or the product patented for the limited period, stimulates new inventions of commercial utility. The price of the grant of the monopoly is the disclosure of the invention at the Patent Office, which after the expiry of the fixed period of the monopoly passes into public domain.
The salient features of the Act (as enacted) were:
-
the reduction of the term of patent from 16 to 14 years;
-
a maximum of seven years for the term of a patent for the processes for drugs and foods;
-
no product patents available for food, drugs and medicines, including the products produced or obtained by chemical processes;
-
provisions prescribing nonworking as a ground for the grant of compulsory licenses, licenses of right and the revocation of patents;
-
the empowerment of government to use inventions for its own use;
-
provisions for the use of inventions for government purposes, research or instruction to pupils;
-
the endorsement of a “license of right” to patents related to drugs, foods and products of chemical reactions;
-
the codification of certain inventions as non-patentable;
-
the expansion of the grounds for opposition to the grant of a patent;
-
exemption from anticipation in respect of certain categories of prior publication, prior communication and prior use;
-
provisions for the secrecy of inventions relevant for defense purposes;
-
the mandatory furnishing of information regarding foreign applications;
-
the prevention of abuse of patent rights by voiding restrictive conditions in license agreements and contracts;
-
a provision for appeal to the High Court from decisions of the Controller General of Patents, Designs and Trade Marks (“the Controller”); and
-
the separation of industrial designs from the law of patents.
However, many provisions changed after the TRIPS Agreement, as discussed in Sections 6.1.4.4.3 to 6.1.4.4.5.
6.1.4 International obligations and commitments
India is a member of the WTO, which came into being on January 1, 1995. The WTO administers the General Agreement on Tariffs and Trade (GATT),16 which is an international agreement among countries to promote free international trade in goods. The WTO is a package deal in that its members must abide by the GATT agreement and a series of other international agreements. One such agreement is the TRIPS Agreement. India is also a member of the Paris Convention for the Protection of Industrial Property,17 the Patent Cooperation Treaty (PCT),18 as well as the Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure.
6.1.4.1 The TRIPS Agreement
TRIPS is one of the most comprehensive multilateral agreements on intellectual property rights.
Section 5 of TRIPS deals with patents. Article 27(1) of TRIPS provides that patents will be available for products or processes of inventions in all fields of technology, provided they are new, involve an inventive step and are capable of industrial application. This was a departure from what the Patents Act, 1970 allowed at the time since no patents were allowed for “substances intended for use, or capable of being used, as food or as medicine or as drug.”19 In such cases, only method or process patents were allowed for such substances.
Article 70(8)–(9) of the TRIPS Agreement stipulates that, during the transition period, a country should provide a mechanism for patent protection for pharmaceutical and agricultural chemical products, including the grant of exclusive marketing rights (EMRs). On July 2, 1995, the U.S. alleged that India had not complied with these provisions. It requested the WTO for dispute consultations. A panel to hear the dispute issued a report on September 5, 1997, finding that India was indeed in violation of these TRIPS Agreement provisions.20 India’s appeal also failed. The appellate body found that, as on January 1, 1995, India was required to have a legal mechanism for patent protection as provided under Article 70(8)–(9) of the TRIPS Agreement.21
In 1997, the European Community requested another dispute consultation on similar grounds. The panel set up in this regard also ruled against India.22 Accordingly, in 1999, India introduced an amendment to comply with these requirements. These, and other amendments of 2002 and 2005, are discussed in Sections 6.1.4.4.3 to 6.1.4.4.5.
6.1.4.2 The Doha Declaration
Prior to the adoption of the TRIPS Agreement, most countries did not grant patents for medicines. This helped keep costs affordable and ensured access to medicines. The introduction of product patents for medicines under the TRIPS agreement was a matter of concern for developing countries and least-developed countries. Increasing the number of product patents for medicines implied that the cost of medications would increase and thwart access to medication.
The TRIPS Agreement required, among other things, that all WTO members introduce product and process patents in all fields of technology. Exceptions in fields related to the fulfillment of basic needs, such as in health, were not recognized or permitted.
In 2001, WTO members adopted a declaration at the WTO Ministerial Conference in Doha, Qatar, stating that it was important to interpret the TRIPS Agreement to support public health by promoting access to medicine and the creation of medicines.23 This was important for developing economies, including India, which had stressed the need to expand public health coverage at low and affordable costs. The Doha Declaration agreed that the TRIPS Agreement did not and should not prevent WTO members from taking measures to protect public health.24
The Doha Declaration recognized that the TRIPS Agreement should be interpreted and implemented in a manner conducive to its members, deploying the flexibilities built into the TRIPS Agreement.25 Consequently, each WTO member was free to determine the grounds on which compulsory licenses were to be granted and what constituted a national emergency or other circumstances of extreme urgency for invoking compulsory licensing provisions.26 The Doha Declaration also recognized that many countries had little or no manufacturing capacity in the pharmaceutical sector and might face difficulties in the effective use of the TRIPS Agreement’s compulsory licensing provisions.27 Pursuant to this, an amendment was accepted in Article 31bis of the TRIPS Agreement, permitting countries to grant compulsory licenses even for export to other countries with insufficient or no manufacturing capacity.
The Doha Declaration also clarified flexibilities for members to adopt an international principle of exhaustion of rights28 in accordance with Article 6 of the TRIPS Agreement.29 The principle of exhaustion means that, once patent holders sell a patented product, they cannot prohibit the subsequent resale of that product, since their rights in respect of that product have been “exhausted” by the act of selling the product. The Doha Declaration reaffirmed that members were free to establish their own regime for such exhaustion to ensure that patent rights did not impede legitimate products entering global supply chains.
6.1.4.3 The Patent Cooperation Treaty
The PCT provides a platform to facilitate the filing of a single international patent application to seek protection across PCT contracting states. This is beneficial for an applicant because, in the traditional system, separate applications for patents had to be made in each jurisdiction across the world. The international search reports and written reports generated by the International Searching Authorities as well as International Preliminary Reports on Patentability (Chapter II) drawn by the International Preliminary Examining Authorities assist the applicant in deciding whether to proceed with the national phase and, if so, in which countries, based on the likelihood of success as per the search report. The PCT system has also resulted in a considerable reduction in costs for applicants.
6.1.4.4 Amendments and implementation in India
6.1.4.4.1 Patent Cooperation Treaty implementation in India
India signed and acceded to the PCT in September 1998, which entered into force in India in December 1998. The provisions relating to applications under the PCT were incorporated under the Patents (Amendment) Act, 2002. Under the Patents Act, 1970, an “international application” was defined as an application made as per the provisions of the PCT.30 Four offices in India (New Delhi, Kolkata, Chennai and Mumbai) and the International Bureau in Geneva, Switzerland were designated as receiving offices for international applications. Section 7 of Act prescribes the form in which an applicant makes an application for its invention and also provides for making a simultaneous application under both the PCT and the Act if a corresponding application has been filed before the Controller in India.31
Chapter III of the Patents Rules, 2003, contains the provisions for filing an international application, the form in which an application is to be made, fees payable to the examining authority, time limits for establishing an international search report and other related rules for applications under the PCT. The term of a patent granted in India for a PCT international application is 20 years from the date of its filing under the PCT.32
6.1.4.4.2 Patent prosecution highway
Apart from the PCT system, several countries and regions have recently created “patent prosecution highways” which provide for accelerated examination, the sharing of search reports and so on, which result in the speedier examination and grant of patents. Such prosecution highways can either be bilateral or multilateral. In India, the first patent prosecution highway was initiated in 2019 by the Indian Patent Office as a bilateral pilot patent prosecution highway program with the Japan Patent Office. Guidelines for the same were published, though the pilot is limited to 100 cases per year, on a first-come-first-served basis. Depending on the evaluation of this pilot highway, long-term patent prosecution highways with one or more patent offices across the country may be a reality.
6.1.4.4.3 The 1999 amendment, post–TRIPS Agreement
Upon coming into effect on January 1, 1995, the TRIPS Agreement provided for transitional periods for WTO members to introduce legislation complying with the obligations under the agreement. India has been a WTO member since January 1995.
For developing countries like India, the deadline for complying with the TRIPS Agreement was the year 2000. Article 65(4) of the TRIPS Agreement provided a special transitional provision for those countries that did not grant product patents. As per this provision, an additional period of five years (i.e., until 2005) on the initial TRIPS Agreement transitional period was permitted for introducing product patent protection.
India needed to provide a means for filing patent applications during the transitional period. The “mailbox provision” allowed applicants to file for patents, thereby establishing filing dates, while at the same time permitting members to defer the granting of product patents. In addition, India also needed to provide EMRs in exchange for permission to delay the grant of product patents until January 1, 2005.
Transitional arrangements were introduced through Section 2 of the Patents (Amendment) Act, 1999, through the insertion of Section 5(2) of the Patents Act, 1970, allowing product patent applications to be filed through a “mailbox,” while Chapter IVA provided for the grant of EMRs if certain conditions were fulfilled
EMRs were introduced as a transitory provision to help developing countries that followed a process patent regime to slowly phase into a product patent regime. In order to bring in transitional measures for the recognition of the TRIPS Agreement obligations, the Patents (Amendment) Act, 1999, introduced a system for the grant of EMRs. This allowed inventors to file early applications for the grant of patents and to establish filing dates so that, when patent protection was ultimately granted, these applications would be considered on the basis of the date of filing or priority dates. These provisions were considered necessary under the TRIPS Agreement,33 pending the initiation of a streamlined process in India for granting product patents relating to drugs, pharmaceutical and agricultural chemical products.
EMRs are applicable where a patent is granted for the same product in another WTO member after 1995 (the date of entry into force of the TRIPS Agreement), provided marketing approval for the product was obtained in such other WTO member. However, EMRs are limited only to pharmaceutical and agricultural chemical products. From a simple dictionary definition of the term, the meaning of “exclusive marketing rights” appears to be very similar to that of patent rights; however, in theory, EMRs prevent others from making or using patented products. The rights holder can indirectly prevent others from marketing products based on such use since they would lack the authorization to do so. Patent protection and EMRs are alternatives to each other and are not used concurrently.
EMRs under the 1999 amendment could only be granted for products intended for or capable of being used as a medicine or drug. For an applicant to have the exclusive right to sell or distribute the product in India, pending the grant or rejection of the application for the product patent, the following conditions needed to be fulfilled:
-
a patent and approval to sell the same invention applied for (on or after January 1, 1995) in another WTO member had been granted after the date of making an application for the product patent;34 or
-
a patent for the method, process or manufacture of the invention applied for (on or after January 1, 1995) and relating to the same product had been granted in India on or after the date of making an application for the product patent;35 and
-
approval to sell or distribute the product had been received from the Central Government.36
EMRs were granted for a period of five years from the date of such approval or until the grant or rejection of the application for the product patent, whichever was earlier.
As per the 1999 amendment, no application for the grant of a product patent could be referred by the Controller to an examiner for making a report until December 31, 2004.37 For the said 10-year period, the applications were kept in a “black box,” a figurative expression for applications pending examination. After this date, the application would be referred to an examiner for a report on whether the claimed invention was within the meaning under Section 3 of the Patents Act, 1970, or whether the invention was such for which no patent could be granted under Section 4 of the Act. If the necessary preconditions were not met, the application would be rejected.38 If the preconditions were fulfilled, the Controller could proceed to consider the application for the grant of a patent.39
The 1999 amendment also included provisions authorizing the Central Government – under expedient circumstances and keeping in mind the public interest at large – to sell or distribute the product by itself or through an authority so empowered in writing.40 Moreover, the Central Government also had the power to direct that the product be sold at a price determined by it after specifying its reasons and the public interest involved.
All suits relating to infringement under Section 24B of the Act would be dealt with in the same manner as suits concerning infringement of patents under Chapter XVIII.
In India, some EMRs were granted relating to medicinal products. Suits for infringement restraining violation of EMR rights were also instituted. However, all EMRs came to an end after the full-scale implementation of the amendments with effect from January 1, 2005. With the introduction of the 2005 amendments, all pending applications for the grant of EMRs were automatically considered as applications for product patents and dealt with accordingly.
6.1.4.4.4 The 2002 amendment, post–TRIPS Agreement
This 2002 amendment to the Patents Act, 1970, was introduced to (a) bring the patent regime in India in line with the TRIPS Agreement; (b) bring the law on patents in line with the increasing development of technological capability of India; (c) provide the necessary safeguards for the protection of public interest and national security; (d) harmonize the procedure for the grant of patents in accordance with the international practices; and (e) make the system more user-friendly.
Some of the salient features of the Patents (Amendment) Act, 2002, were as follows:
-
The term of every patent granted after the commencement of the Patents (Amendment) Act, 2002, was increased to 20 years from the date of filing of the application.41
-
The time for restoration of a lapsed patent was increased to 18 months.42
-
A new definition for “invention” was added: a patent could be for a process or product that was new, involved an inventive step or was capable of industrial application.43
-
A new definition for “inventive step” was added.44
-
The negative list of what were not considered inventions (i.e., non-patentable subject matter) was amended and expanded in light of Article 27(2)–(3) of the TRIPS Agreement.45
-
The concept of a request for the publication of a patent application was introduced.46
-
An onus-of-proof provision was introduced, requiring the defendant to prove that its process for obtaining the product in question was different from the patented process in cases where an identical final product was obtained from such a process.47
-
The chapter on compulsory licensing was substituted with provisions and procedures consistent with the TRIPS Agreement,48 and the provisions relating to the license of rights were omitted.49
-
The Bolar exemption was introduced.50
-
The parallel import of patented products was introduced.51
-
All appeals under the Act were redirected from the High Courts to a specialized tribunal (i.e., the Intellectual Property Appellate Board (IPAB)52 since abolished in 2021).53
-
National security provisions were amended.54
6.1.4.4.5 The 2005 amendment, post–TRIPS Agreement
The amendments of 2005 were introduced to bring Indian patent laws into further compliance with the TRIPS Agreement because the transitional period available to India was ending in 2005. Some of the salient features of the Patents (Amendment) Act, 2005, were as follows:
-
The definition of “inventive step” was amended.55
-
The definition of “new invention” was added.56
-
The definition of “patent” was amended.57
-
The negative list of what were not considered inventions (i.e., non-patentable subject matter) was amended.58
-
The provisions that provided that only the process and not the product itself would be patented in cases of inventions relating to food, drugs and medicines were deleted.59 This ensured that product patent protection was available for all fields.
-
The chapter relating to EMRs was omitted,60 and the provisions relating to it were modified.
-
Two levels of opposition were introduced – pre-grant and post-grant. All grounds available for pre-grant opposition were also made available to interested parties for challenging a patent in post-grant opposition within one year from the date of publication of the grant of patent.61
-
Pursuant to the Doha Declaration, the grounds for seeking compulsory licensing were expanded by adding a provision for the issuance of compulsory licenses for the manufacture and export of patented pharmaceutical products to countries that had insufficient manufacturing capacity in the pharmaceutical sector if that country had allowed such importation by notification.62
-
Jurisdiction for trying revocation petitions to revoke granted patents was shifted from the High Courts to the IPAB with a view to extending its jurisdiction to the revocation of patents.63 This now stands reversed in 2021.64
-
Certain provisions were amended to bring the patent regime in India in line with the PCT, to which India is a signatory.65
6.1.5 Patent application trends
Figure 6.1 shows the total number of patent applications (direct and Patent Cooperation Treaty (PCT) national phase entry) filed in India from 2000 to 2021.
Figure 6.1 Patent applications filed in India, 2000–2021
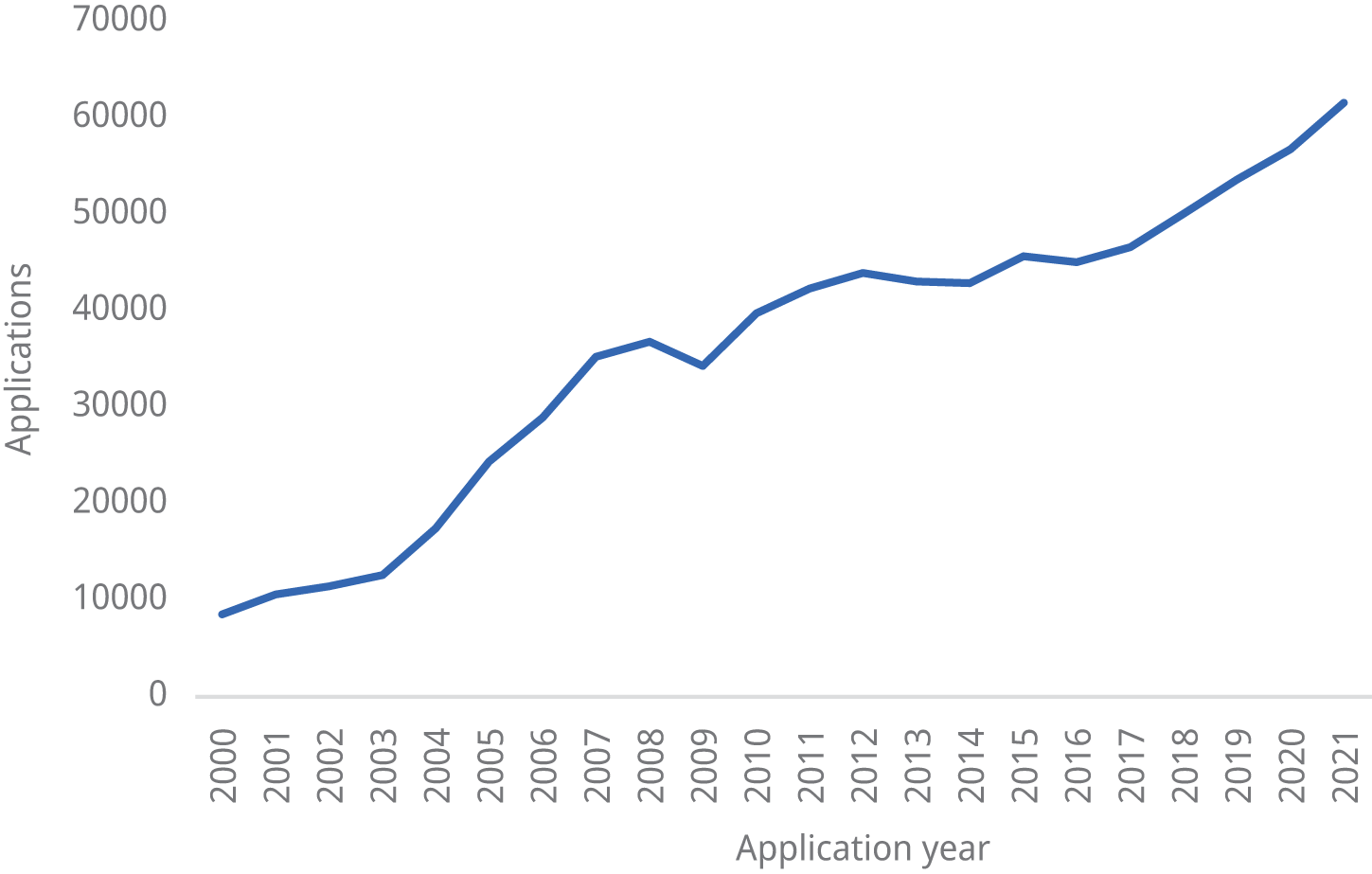
Source: WIPO IP Statistics Data Center, available at www3.wipo.int/ipstats/index.htm?tab=patent
6.2 Patent institutions and administrative review proceedings
6.2.1 Patent institutions
6.2.1.1 Office of the Controller General of Patents, Designs and Trade Marks
The Office of the Controller General of Patents, Designs and Trade Marks is located in Mumbai. The Controller supervises the working of the Patents Act, 1970, the Designs Act, 2000, and the Trade Marks Act, 1999, and also renders advice to the Government on matters relating to these subjects.
The Central Government may appoint as many examiners and other officers with such designations as it thinks fit.66 Minimum qualifications are prescribed. These officers function under the Controller’s superintendence. Higher qualifications are prescribed for the position of Senior Joint Controller of Patents and Designs. The organizational structure of the Office is shown in Figure 6.2.
Figure 6.2 Organizational structure of the Office of the Controller General of Patents, Designs and Trade Marks
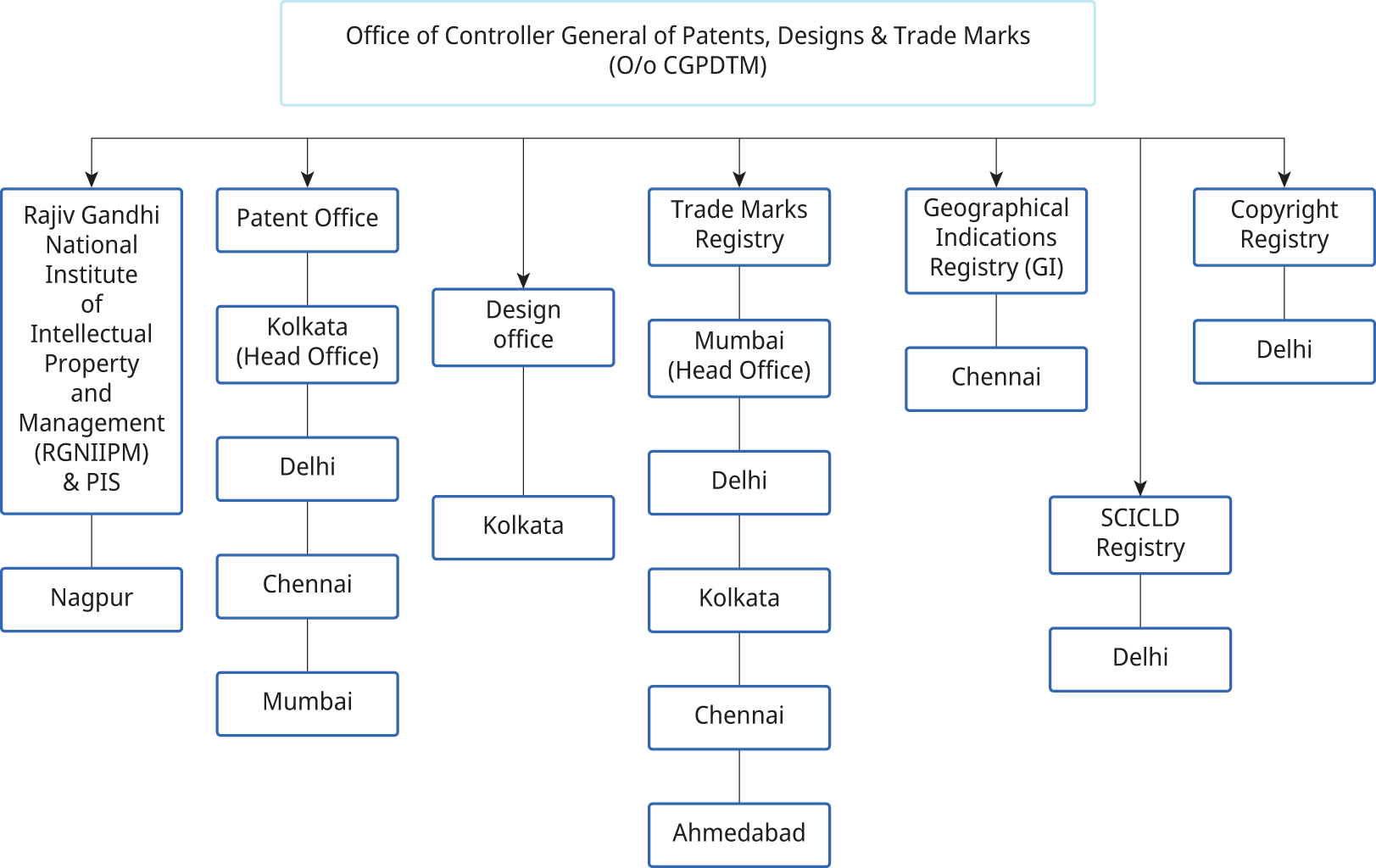
Source: Office of the Controller General of Patents, Designs and Trade Marks, About Us, ipindia.gov.in/about-us.htm
6.2.1.2 The Department for Promotion of Industry and Internal Trade
The Department for Promotion of Industry and Internal Trade was established in 1995 and reconstituted in 2000 when it was merged with the Department of Industrial Development. The department’s purpose is to promote and accelerate the industrial development of the country by facilitating investment in new and upcoming technologies, foreign direct investment and supporting the balanced development of industries.
The department is the nodal department for all matters related to the protection of IP rights in the fields of patents, trademarks, copyrights, industrial designs and geographical indications.
6.2.1.3 National Institute of Intellectual Property Management, Nagpur
The National Institute of Intellectual Property Management is a national center for excellence in training, management, research and education in IP rights. The institute trains examiners of patents and designs, examiners of trademarks and geographical indications, IP professionals and IP managers in the country. The institute also facilitates research on IP-related issues.
The Patent Information System was established by the Indian Government in 1980 to maintain a comprehensive collection of patent specifications and patent-related literature worldwide. It is also located in Nagpur within the premises of the National Institute of Intellectual Property Management.
6.2.1.4 Cell for IPR Promotion and Management, constituted under the National Intellectual Property Rights Policy
The Cabinet of Ministers of the Central Government approved the National Intellectual Property Rights Policy on May 12, 2016.67 This policy drew a future roadmap for IP rights in India and made several recommendations. Following one of the recommendations of the 2016 policy, a specialized professional body – the Cell for IPR Promotion and Management – was created under the aegis of the Department for Promotion of Industry and Internal Trade, and it has been instrumental in taking forward the objectives and visions of the policy. Since the adoption of the policy, the cell has worked toward changing the IP landscape of the country, which among other things, has included:
-
IP rights awareness programs, which are conducted in over 200 academic institutions for the industry, police, customs and the judiciary;
-
reaching out to rural areas – awareness programs are being conducted using satellite communication (EduSat). In one such program, 46 rural schools, with a combined total of 2,700 students, were reached. Over 300 schools and more than 12,000 students have been reached;
-
more focus on developing e-content and disseminating content through online channels;
-
including content on IP rights in the National Council of Educational Research and Training commerce curriculum. Work is ongoing to include IP rights in other academic streams; and
-
conducting competitions in conjunction with industry for school and college students to develop the “innovative spirit.” Some competitions have included the development of mobile applications, videos and online games.
As part of the awareness campaign, the Cell for IPR Promotion and Management also launched India’s first IP mascot – “IP Nani” – in collaboration with the European Union Intellectual Property Office. IP Nani is an animated grandmother who sends out messages for the protection and enforcement of IP. There are also a series of animated videos on IP rights for school students.68 These videos are available for viewing on platforms such as YouTube.69
6.2.1.5 The Department of Science and Technology – Patent Facilitation Programme
The Department of Science and Technology, under the Ministry of Science and Technology, has been implementing its Patent Facilitation Programme since 1995. It has established a Patent Facilitating Cell at the Technology, Information, Forecasting Assessment Council (an autonomous body of the department) and, subsequently, 26 patent information centers in various states. The patent facilitating cells and patent information centers create awareness of and extend assistance in protecting IP rights at the state level, including for patents, copyright, industrial designs, geographical indications and so on.
These patent information centers have also established IP cells in universities in their respective states to enlarge the network. Today, more than one hundred such cells have been created in different state universities. In addition, these centers are also mandated to provide assistance to inventors from government organizations and from central and state universities. They also render ongoing technical and financial assistance in filing, prosecuting and maintaining patents on behalf of the Government, research and development institutes, and academic institutions.70 The mandate of the program is:
-
providing patent information as a vital input to research and development;
-
facilitating patent and IP rights facilitation for academic institutions and Government research and development institutions;
-
providing IP rights policy input to the Government; and
-
conducting IP rights training and awareness programs in the country.
6.2.1.6 Traditional Knowledge Digital Library
The Traditional Knowledge Digital Library (TKDL) is a pioneering initiative in India to protect Indian traditional medicinal knowledge and prevent its misappropriation. It was set up in 2001 as a collaboration between the Council of Scientific and Industrial Research and the Ministry of Ayush, Government of India. The Council of Scientific and Industrial Research is a contemporary research and development organization and a pioneer in India’s IP movement.
The TKDL has overcome the language and format barrier by systematically and scientifically converting and structuring the available contents of ancient texts on Indian systems of medicine (i.e., Ayurveda, Siddha, Unani, Sowa Rigpa and Yoga) into five international languages – English, Japanese, French, German and Spanish – with the help of information technology tools and an innovative classification system called the Traditional Knowledge Resource Classification. More than 360,000 formulations and practices have been transcribed into the TKDL database.
The classification has also structured and classified the Indian traditional medicine system into several thousand subgroups for Ayurveda, Unani, Siddha and Yoga. The Traditional Knowledge Resource Classification has enabled the incorporation of about 200 subgroups under International Patent Classification A61K 36/00, more than the few subgroups earlier available on medicinal plants under A61K 35/00, thus enhancing the quality of search and examination of prior art for patent applications in the area of traditional knowledge.
The TKDL has also established international specifications and standards for setting up traditional knowledge databases based on TKDL specifications. These standards were adopted in 2003 by the committee in the fifth session of the World Intellectual Property Organization’s Intergovernmental Committee on Intellectual Property and Genetic Resources, Traditional Knowledge and Expression of Folklore.
Currently, the TKDL is based on open-source and open-domain texts of Indian systems of medicine. The TKDL acts as a bridge between these books (prior art) and patent examiners. Access to the TKDL is available to 13 patent offices under the TKDL Access (Non-disclosure) Agreement,71 which has inbuilt safeguards on nondisclosure to protect India’s interest against any possible misuse.
The TKDL is proving to be an effective deterrent against biopiracy and has been recognized internationally as a unique effort. It has set a benchmark in traditional-knowledge protection around the world, particularly in traditional-knowledge-rich countries, by demonstrating the advantages of proactive action and the power of strong deterrence. The key here is preventing the grant of incorrect patents conferring monopolies on aspects of traditional knowledge, by ensuring access to prior art relating to traditional knowledge for patent examiners without restricting the use of that traditional knowledge.
6.2.2 Administrative review proceedings
6.2.2.1 Intellectual Property Appellate Board
Under the Patents Act, 1970, the appellate jurisdiction to hear appeals and the original jurisdiction to revoke patents was conferred on the High Courts in India. Both these jurisdictions were redirected to the IPAB as a specialized IP tribunal in 2002 and 2005. This was to enable the speedy disposal of such matters (see Figure 6.3 regarding the disposal of cases by the IPAB).72 However, in 2021, the Central Government was of the view that this stated objective of speedy disposal was not being achieved and abolished the IPAB, redirecting such matters back to the High Courts.73
Figure 6.3 Disposal of cases at the Intellectual Property Appellate Board, up to February 13, 2021
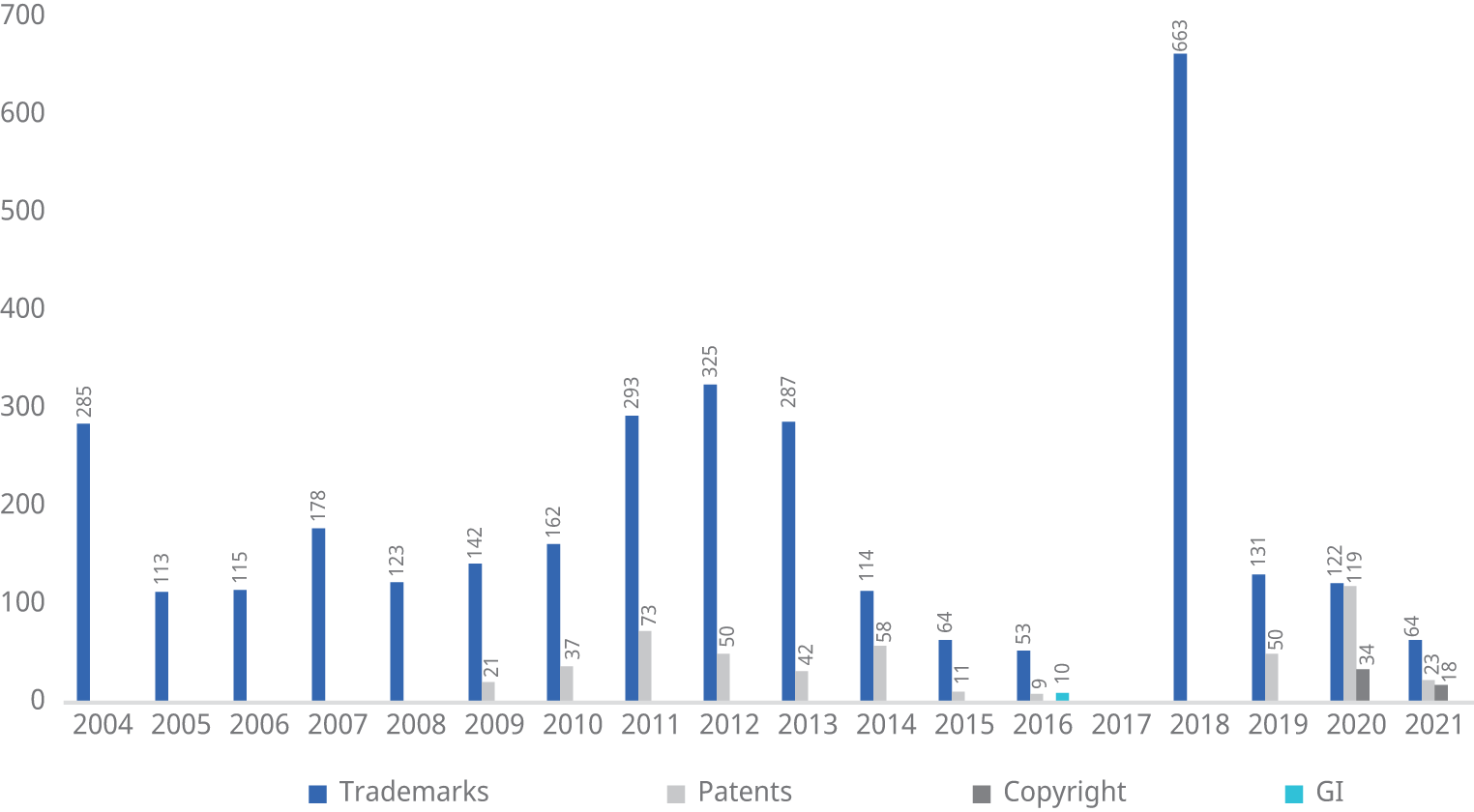
Note: GI = geographical indication.
Source: Jacob Schindler, Top Judge’s Blueprint for the Future in IP Litigation in India, IAM Media (May 5, 2021), www.iam-media.com/law-policy/specialised-ip-bench-in-india-long-overdue-says-delhi-high-court-veteran
6.2.2.2 Pre-grant opposition
The scheme of pre-grant oppositions was streamlined by the Patents (Amendment) Act, 2005. Prior to this, a pre-grant opposition could only be filed by a “person interested.” The amendment now allows any person to file a pre-grant opposition. It can be filed when an application for a patent has been published, but the patent has not yet been granted under Section 25(1) of the Patents Act, 1970. There is no time limit within which a pre-grant opposition must be filed after publication.
6.2.2.2.1 Procedure of pre-grant opposition
The pre-grant opposition procedure broadly follows these steps:
- 1. The pre-grant opposition is filed, along with evidence, if any.74
- 2. The Controller forms a prima facie opinion on the pre-grant opposition filed. They decide either to issue notice of the opposition to the patent applicant or to reject the opposition prima facie without issuing notice to the patent applicant.75
- 3. If notice has been issued, the patent applicant may reply (along with evidence, if any) within three months from the date of the notice by the Controller.76
- 4. The Controller may hold a “hearing.” This is followed by a decision, ordinarily within one month.77 The Controller is required to either reject or grant the patent.
6.2.2.2.2 Grounds on which pre-grant opposition can be filed
Section 25(1) of the Patents Act, 1970, provides a list of grounds on which a pre-grant opposition can be filed. The list is exhaustive:
- (a) that the applicant for the patent or the person under or through whom he claims, wrongfully [emphasis added] obtained the invention or any part thereof from him or from a person under or through whom he claims;
-
(b) that the invention so far as claimed in any claim of the complete specification has been published before the priority date of the claim –
- (i) in any specification filed in pursuance of an application for a patent made in India on or after the 1st day of January, 1912; or
-
(ii) in India or elsewhere, in any other document:
Provided that the ground specified in sub-clause (ii) shall not be available where such publication does not constitute an anticipation of the invention by virtue of sub-section (2) or subsection (3) of section 29;
- (c) that the invention so far as claimed in any claim of the complete specification is claimed in a claim of a complete specification published on or after priority date of the applicant’s claim and filed in pursuance of an application for a patent in India, being a claim of which the priority date is earlier than that of the applicant’s claim;
-
(d) that the invention so far as claimed in any claim of the complete specification was publicly known or publicly used in India before the priority date of that claim.
Explanation. – For the purposes of this clause, an invention relating to a process for which a patent is claimed shall be deemed to have been publicly known or publicly used in India before the priority date of the claim if a product made by that process had already been imported into India before that date except where such importation has been for the purpose of reasonable trial or experiment only;
- (e) that the invention so far as claimed in any claim of the complete specification is obvious and clearly does not involve any inventive step, having regard to the matter published as mentioned in clause (b) or having regard to what was used in India before the priority date of the applicant’s claim;
- (f) that the subject of any claim of the complete specification is not an invention within the meaning of this Act, or is not patentable under this Act;
- (g) that the complete specification does not sufficiently and clearly describe the invention or the method by which it is to be performed;
- (h) that the applicant has failed to disclose to the Controller the information required by section 8 or has furnished the information which in any material particular was false to his knowledge;
- (i) that in the case of a convention application, the application was not made within twelve months from the date of the first application for protection for the invention made in a convention country by the applicant or a person from whom he derives title;
- (j) that the complete specification does not disclose or wrongly mentions the source or geographical origin of biological material used for the invention;
- (k) that the invention so far as claimed in any claim of the complete specification is anticipated having regard to the knowledge, oral or otherwise, available within any local or indigenous community in India or elsewhere, but on no other ground, and the Controller shall, if requested by such person for being heard, hear him and dispose of such representation in such manner and within such period as may be prescribed.
6.2.2.2.3 Locus standi to file pre-grant oppositions
Under Section 25(1) of the Patents Act, 1970, “any” person can file a pre-grant opposition. A pre-grant opposition is deemed to be an extension of the examination by the Patent Office, and, thus, the standing requirement is diluted. Nevertheless, precedents demonstrate that courts come down heavily against fake pre-grant oppositions or those filed by impostors solely to harass or to delay the grant rather than with any genuine intent to remove invalid patents.78
6.2.2.3 Post-grant opposition
A post-grant opposition can be filed under Section 25(2) of the Patents Act, 1970, by a “person interested” after the grant of patent but within one year from the date of publication of the grant of a patent.
6.2.2.3.1 Procedure in filing post-grant opposition
The post-grant opposition procedure broadly follows these steps:
- 1. A post-grant opposition is filed, along with evidence, if any.79 A copy must be supplied to the patentee.80
- 2. The Controller constitutes an opposition board of three examiners (other than the examiner who examined the patent).81
- 3. The patent applicant may reply to the opposition, providing evidence, if any, within two months.82 If no reply is filed, the patent is deemed to be abandoned.83 The Controller also notifies the patentee.84 The time for this reply begins from the date the patent applicant is served with the opposition by the opponent.
- 4. The opponent then has one month to respond to the patent applicant’s reply statement.85
- 5. The opposition board prepares a report with reasons on each ground taken in the notice of opposition. The report contains the board’s joint recommendation and is made within three months of the date on which the documents were forwarded to the board.86
- 6. The Controller schedules a hearing. This is followed by a decision.87 At the hearing, the Controller may require members of the opposition board to be present. If either of the parties wishes to be heard, this is permitted on payment of a fee and after notice. If no notice to attend the hearing is received from either party, the Controller can decide the opposition without a hearing. The order must be reasoned. The recommendation of the opposition board is not binding, though it is of persuasive value. Thus, the board’s recommendation should not be lightly ignored without stated reasons.
A party can also file new documents before the scheduling of the hearing, provided prior leave of the Controller is obtained.88 Further, a party can rely upon “any publication” that may not have been filed earlier, provided that there has been five days’ notice and that the details of the publication are given to the other party.89
In Cipla Ltd v. Union of India, the Supreme Court held that it would be mandatory to issue a copy of the recommendation of the Opposition Board to the parties, so that the principles of natural justice are duly adhered to.90
6.2.2.3.2 Grounds on which post-grant opposition can be filed
The grounds for post-grant opposition are the same as those for pre-grant opposition. The grounds are exhaustive.
6.2.2.3.3 Locus standi to file post-grant oppositions
Section 2(1)(t) of the Patents Act, 1970, defines “person interested” in an inclusive manner to include a person engaged in or promoting research in the same field as that to which the invention relates. Precedents have interpreted the term “person interested” broadly to cover any person who has a direct, present and tangible interest in the patent and those whose interests are adversely affected because of the patent.91 The term has been construed to include even nongovernmental organizations that have an interest or stake in the existence or invalidity of the patent – commercial interest is not a necessary condition.92
6.2.2.3.4 Appeals from pre-grant and post-grant oppositions
An appeal lies to the jurisdictionally competent High Court from an order of rejection of a patent application in a pre-grant opposition and from an order revoking or maintaining the grant of patent in a post-grant opposition. No appeal lies from the grant of a patent in a pre-grant opposition.93
6.3 Judicial institutions
6.3.1 Court system in India
6.3.1.1 Hierarchy of courts
There is a common court structure across India, with the Supreme Court of India at its apex. The Supreme Court of India is a court established under the Constitution of India. It is located in New Delhi and is the final appellate authority in the Indian judicial system. The Supreme Court has appellate, constitutional, review and special jurisdictions. It also has limited original jurisdiction for constitutional matters, though not for IP matters.
Below the Supreme Court of India are the various High Courts of India. The Supreme Court exercises appellate jurisdiction over High Court decisions. However, all High Courts and the Supreme Court of India occupy equal constitutional status. While, typically, each federal Indian state has a designated High Court, some states share a High Court. There are 24 High Courts in India.
All High Courts have appellate, constitutional and review jurisdiction. A few High Courts also have “original” jurisdiction – civil cases, including IP suits, can be directly filed in these High Courts, subject to a certain minimum pecuniary value that may vary from one state to another. Such High Courts are those of Delhi, Bombay (Mumbai), Madras (Chennai), Calcutta (Kolkata) and Himachal Pradesh (Shimla). All appeals from a High Court lie to the Supreme Court, though some High Courts also possess an intracourt appeal system from a single judge of the High Court to a bench comprising two judges (i.e., division bench).94
Each federal Indian state is typically divided into several districts. Below the High Courts of each state are the district and sessions courts for each such district. The district court is for civil matters, and the sessions court is for criminal matters. Below these courts are the courts of sub-judges for civil matters and the magistrates’ courts for criminal matters.
6.3.1.2 Commercial courts
The Commercial Courts Act, 2015, was enacted to provide fast-track courts for the resolution of certain commercial disputes, which includes IP rights disputes. All commercial disputes beyond a certain minimum specified value must be filed under the fast-track system of the Commercial Courts Act, 2015. Each district now has designated commercial courts for such disputes. Each High Court having original jurisdiction also has a Commercial division to hear such fast-tracked commercial disputes. Further, every High Court has a Commercial Appellate division to hear appeals for fast-tracked commercial disputes.
6.3.1.3 Appointment and tenure of judges
Judges of the High Courts and the Supreme Court of India are selected by a committee (called “the Collegium”) consisting of the three or five seniormost judges of the Supreme Court and headed by the Chief Justice. The executive can give its views on specific candidates, though the Collegium has the final say. A High Court judge could be from the district judiciary95 (or a practicing advocate with a minimum of 10 years’ practice).
Appointments to the subordinate judiciary (i.e., lower than the district court: the Provincial Civil Service–Judicial) are made by either the state public service commissions or the High Court concerned. The selection process involves written tests and an interview. Selected candidates are appointed as judges in the subordinate judiciary as sub-judges. High Courts also conduct the selection process for the Higher Judicial Service’s appointment of district judges. Candidates for the Higher Judicial Service are sub-judges and advocates with a minimum of seven years’ practice.
6.3.2 Judicial education on intellectual property
The National Judicial Academy (NJA) is a training institute located in Bhopal, Madhya Pradesh, established and fully funded by the Government of India. The NJA is an independent society established in 1993 under the Societies Registration Act, 1860. The Honorable Chief Justice of India is the Chairman of the General Body of the NJA and the Chairman of the Governing Council, the Executive Committee and the Academic Council of the NJA.
The NJA’s academic programs are guided by the National Judicial Education Strategy, launched in 2007. Under this strategy, the NJA has established a national system for judicial education. The NJA conducts a vibrant training program for judges at all levels throughout the year. The program is addressed by speakers who may be lawyers or people with specialized knowledge, including economists and foreign experts.
As a joint initiative of the World Intellectual Property Organization and the NJA, seminars, talks and so on are organized by the NJA for the benefit of lawyers, academics and students.
6.4 Challenges to patents
Under Section 13(4) of the Patents Act, 1970, the grant of a patent does not guarantee its validity.96 The underlying principle is that allowing an invalid patent to continue on the register is against the public interest, so every opportunity is provided to remove invalid patents. There are various levels of challenges provided in the Act against a patent application or a granted patent. Such challenges can be made either prior to or after the grant:
-
Pre-grant opposition under Section 25(1);
-
Post-grant opposition under Section 25(2) before the Patent Office, introduced in 2005;
-
revocation under Section 64(1) before the High Court;97 and
-
a counterclaim seeking revocation in a suit for infringement under Section 64(1), in which case the infringement suit and the counterclaim are both transferred to the relevant High Court.98
Other challenges to patents or the exercisable rights are compulsory licenses and government use (under Sections 84, 92, 102 and others) and revocation (under Section 66).
There has been significant discontent – especially after the 2002 and 2005 amendments – about the multitude of challenge avenues. These provisions for patent challenges may appear to encourage abuse by patent opponents and result in patent grants being held up or delayed almost indefinitely. These apprehensions have been assuaged to a large extent by judicial precedents, which have streamlined the filing of oppositions and dealt with challenges to orders passed in oppositions. In UCB Farchim v. Cipla Ltd,99 the Delhi High Court confirmed that, once a pre-grant opposition is dismissed and the patent is granted, the order granting the patent cannot be challenged by way of an appeal. The only remedy available is to file a post-grant opposition or a revocation. In Snehlata C Gupte v. Union of India,100 the practice of filing serial oppositions to hold up the grant of a patent was stopped by the Delhi High Court. The court issued a series of directions preventing delays in patent grants. In Aloys Wobben v. Yogesh Mehra,101 the Supreme Court categorically held that one person cannot pursue both a revocation application and a counterclaim seeking revocation. These and other decisions have ensured that duplicity and parallel proceedings are avoided to the extent possible.
6.5 Patent infringement
The ability to enforce patents is a crucial right for any patentee. Chapter XVIII of the Patents Act, 1970, addresses infringement, forums, remedies, defenses and counterclaims.
6.5.1 Claim construction
Claim construction forms a critical component of patent enforcement and invalidity challenges. Claim construction is a prerequisite for infringement analysis because the claims determine the scope of protection afforded to the patentee. Similarly, only after claims are construed to determine the invention can invalidity analysis proceed.
6.5.1.1 Procedure
Unlike the mechanism of a “Markman” hearing in the U.S., there is no separate procedural step for claim construction. Instead, claim construction is handled as part of the trial. Any disputes concerning the construction of claims will be framed as issues during the case management hearing. In the High Court of Delhi Rules Governing Patent Suits, 2022, it has been recommended that parties file a claim-construction brief before the case management hearing to enable courts and parties to assess whether there are any disputes in relation to the claims.102
6.5.1.2 Principles
In the context of India’s predecessor legislation, the Supreme Court of India has held that claims must be given an effective meaning and that the specification and claims must be examined and construed together.103 The Supreme Court followed English precedents when coming to this conclusion.
Under the Patents Act, 1970, the Delhi High Court, in F Hoffmann-La Roche Ltd v. Cipla Ltd,104 held that one must undertake a “purposive construction” of the claims. The Delhi High Court drew inspiration from the concept of purposive construction that was formulated in the seminal English judgment Catnic Components Ltd v. Hill and Smith Ltd105 This principle is captured in the following two dicta in the Catnic Components case:
whether persons with practical knowledge and experience of the kind of work in which the invention was intended to be used, would understand that strict compliance with a particular descriptive word or phrase appearing in a claim was intended by the patentee to be an essential requirement of the invention so that any variant would fall outside the monopoly claimed, even though it could have no material effect upon the way the invention worked.
No plausible reason has been advanced why any rational patentee should want to place so narrow a limitation on his invention. On the contrary, to do so would render his monopoly for practical purposes worthless, since any imitator could avoid it and take all the benefit of the invention by the simple expedient of positioning the back plate a degree or two from the exact vertical.106
This principle of purposive construction was streamlined in the form of “Improver” questions in a subsequent judgment in the United Kingdom (U.K.)107 and later approved by the House of Lords.108 However, the U.K. Supreme Court, in Actavis UK Ltd v. Eli Lilly and Co.,109 disagreed with the earlier line of cases. According to the U.K. Supreme Court, this earlier line of case law on purposive construction conflated the issue of claim construction and infringement analysis.
The current standard in the U.K. requires a court to adopt a “normal interpretation” approach. For infringement purposes, according to the U.K. Supreme Court, one must examine whether the infringing device or process infringes the claim as construed by such normal interpretation. If not, the U.K. Supreme Court dictates that a court must thereafter assess whether the claim is infringed by equivalents. It has formulated a test for assessing such equivalents. The U.K. Supreme Court’s judgments in Actavis UK Ltd and subsequently in Icescape Ltd v. Ice-World International BV 110 have clarified that the normal interpretation of claims is also purposive. Such interpretations are purposive because courts are to construe claims as per their ordinary language, in their context (description and drawings) and in the light of the factual background (common general knowledge in the art).
There has been no subsequent judgment in India addressing these jurisprudential developments. However, since even the earlier rulings of the Supreme Court of India and the Delhi High Court were guided by the English precedents, it is expected that Indian courts will take a similar approach to claim construction.
6.5.2 Infringement analysis
6.5.2.1 What is “infringement”?
The Patents Act, 1970, does not separately define “infringement,” but courts regard any violation of the rights accorded under Section 48 of the Act as an infringement. Like most international jurisdictions, and consistent with Article 28 of the TRIPS Agreement, Section 48 of the Act confers an exclusive right on the patentee to prevent third parties from “making, using, offering for sale, selling or importing for those purposes” the patented product.111 In the case of process patents, the patentee has the exclusive right to prevent third parties from using the process and from “using, offering for sale, selling or importing for those purposes” the “product obtained directly by the patented process.”112 Committing these acts without the patentee’s consent constitutes infringement.
6.5.2.2 Exports as infringement
The Delhi High Court has held that the term “sale,” in the context of another provision of the Patents Act, 1970, includes “exports.”113 The Delhi High Court has also recently granted an interim injunction because exports from India would have also amounted to use in India.114
6.5.2.3 Proving infringement
A plaintiff must compare the alleged infringing product or process with the granted claim or claims to prove infringement.115 Claim construction precedes this exercise of comparison.116
The Patents Act, 1970, is silent on the doctrine of equivalence and other analogous concepts. The predecessor legislation allowed patentees to sue for infringement even when the infringers counterfeited or imitated the invention.117 Case law under the predecessor legislation suggested that courts would ignore “trifling or unessential variation.”118 Defendants were guilty of infringement if they made “what is in substance the equivalent of the patented article.”119 Case law under the current Act suggests that a similar approach may be followed.120
6.5.3 Defenses to infringement
The rights under Section 48 of the Patents Act, 1970, are expressly subject to other provisions of the Act. Section 107(1) states that all the grounds for revoking a patent for invalidity can be used as defenses to a claim for patent infringement. Defendants can, thus, contest the suit patent’s validity even without filing a counterclaim.
Section 107A recognizes the Bolar exception for defendants to use the patented product or process for developing information for regulatory filings both in India and abroad. The Delhi High Court’s judgment in Bayer Corp. v. Union of India121 carries a detailed discussion of the Bolar exception.
India follows the rule of international exhaustion regarding patents. Under Section 107A(b) of the Act, importing a duly authorized product from a foreign jurisdiction is not an infringement. Thus, the position is similar to that under the Trade Marks Act, 1999,122 but different from the domestic exhaustion rule followed under the Indian Copyright Act, 1957.123
Section 107(2), read with Section 47, contains well-known exclusions from the scope of a patent’s exclusivity, such as the research exemption, educational use and governmental use.
6.5.4 Counterclaim of invalidity
Defendants invariably file a counterclaim seeking revocation under Section 64(1) of the Patents Act, 1970. The grounds provided for revocation under Section 64(1) are exhaustive. There is a view that courts retain the discretion not to revoke a patent despite the fulfillment of one or more of the grounds under Section 64(1),124 though this does not seem to be the correct position in law. Proving any one of the grounds under Section 64(1) ought to lead to revocation of the patent.
The grounds for revocation usually taken in a counterclaim include lack of novelty or inventive step and non-patentable subject matter. It is also usual for defendants to support the grounds for revocation, especially in respect of lack of novelty and inventive step, by relying upon claims granted in other jurisdictions. If, in any other foreign jurisdiction, claims granted in corresponding patents are narrower than those granted in India, it is common for defendants in India to question the validity of the Indian patent by referring to such claims. Thus, it is advisable for patentee-plaintiffs in infringement actions in India to check whether the scope of claims in other significant jurisdictions differs, at least broadly, from that of the claims in India. If the patentee narrows the claims in other jurisdictions, it is advisable to make similarly narrower claims in India at the prosecution stage.
The citing of corresponding claims from foreign jurisdictions relates to the concept of “file-wrapper estoppel.” Although patent rights are strictly territorial, defendants argue that the patentee ought to be bound by statements, concessions and amendments made by the patentee before foreign patent offices concerning the same invention. Usually, such narrowing amendments in foreign jurisdictions, without corresponding Indian amendments, could adversely impact the grant of interim relief.
Another ground that defendants often rely upon is noncompliance with Section 8 obligations. Section 8(1) of the Act requires mandatory disclosure of the details of all corresponding foreign applications. Section 8(2) requires the filing of the prosecution history of corresponding foreign applications if so directed by the Indian Patent Office.
An issue frequently agitated in Indian courts, in invalidity challenges to pharmaceutical patents, concerns coverage and disclosure. In Novartis AG v. Union of India,125 the Supreme Court held that a patentee cannot contend that a patent’s coverage is more expansive than its disclosure. The following observation by Justice Jacob in the English case of European Central Bank v. Document Security Systems Inc. is often cited in the Indian context:
Professor Mario Franzosi likens a patentee to an Angora cat. When validity is challenged, the patentee says his patent is very small: the cat with its fur smoothed down, cuddly and sleepy. But when the patentee goes on the attack, the fur bristles, the cat is twice the size with teeth bared and eyes ablaze.126
Since claims are granted only upon an enabling disclosure, courts must presume that a prior patent discloses the claimed subject matter in an enabling manner. However, there have been various opinions expressed that the patent coverage could be wider than the disclosure, leading to multiple patents thereafter. The Delhi High Court has recently considered this issue in a series of interim orders, wherein the preponderance of the view favored the interpretation in Novartis.127 This view is presently the prevalent one.
6.6 Judicial patent proceedings and case management
6.6.1 Key features in patent proceedings
As in all civil cases, the onus of proving infringement is on the plaintiff suing for infringement.128 The court may shift the evidentiary burden and call upon the defendants to establish the noninfringement of process claims in specific circumstances consistent with Article 34 of the TRIPS Agreement. Section 104A of the Patents Act, 1970, provides for two situations in which the defendant can be asked to prove noninfringement of a process claim. One condition precedent common to both situations is that the defendant’s product must be identical to the product directly obtained by the patented process. Once this condition is fulfilled, the court retains the power to demand that the defendant prove noninfringement if the process is for obtaining a new product129 or if the plaintiff shows a substantial likelihood that the defendant is using the patented process and is unable to determine the defendant’s process despite reasonable efforts.
The court may not require the defendant to disclose its process if such disclosure would result in the disclosure of any trade, manufacturing or commercial secrets that form part of the defendant’s process, but only if the disclosure appears reasonable to the court.130 The use of confidentiality clubs, however, may aid even in such disclosure.131
6.6.2 Forum and locus standi to initiate infringement actions
A patent enforcement action under Section 104 of the Patents Act, 1970, can be initiated before a district court or higher. The court will try a patent suit as a commercial suit under the Commercial Courts Act, 2015.132 This also applies to a suit seeking a declaration of noninfringement.133 However, if a defendant in an infringement action counterclaims the patent’s invalidity, the suit and the counterclaim are automatically transferred to the High Court for further adjudication.134 A declaratory suit for noninfringement cannot question the patent’s validity.135The registered owner of the patent, or the assignee thereof, is entitled to sue for infringement. Section 109 of the Patents Act, 1970, provides that an exclusive licensee may sue for patent infringement but must implead the patent’s registered owner as a defendant. Under Section 110 of the Act, a person who has been granted a compulsory license may also sue for patent infringement if, upon notification of infringement to the patentee, the patentee fails to take action within two months.
6.6.3 Early case management
Once all pleadings are complete, the suit is listed before a designated commercial court single-judge bench in a case management hearing for framing issues. The court identifies, as precisely as possible, the issues that arise for determination; directs the filing of witness statements; and sets the schedule for trial. The Commercial Courts Act, 2015, prescribes short time limits for completing pleadings. Pending interim applications do not (and should not) delay the case management hearing for framing issues.
6.6.3.1 Pleadings and overall case schedule
The Commercial Courts Act, 2015, fixes mandatory timelines for filing all pleadings. The Supreme Court of India, in SCG Contracts India Pvt. Ltd v. KS Chamankar Infrastructure Pvt. Ltd,136 confirmed that the timelines fixed under the Act are mandatory. The Act also prescribes a schedule for the entire case (see Figure 6.4).
Figure 6.4 Overall case schedule according to the Commercial Courts Act, 2015
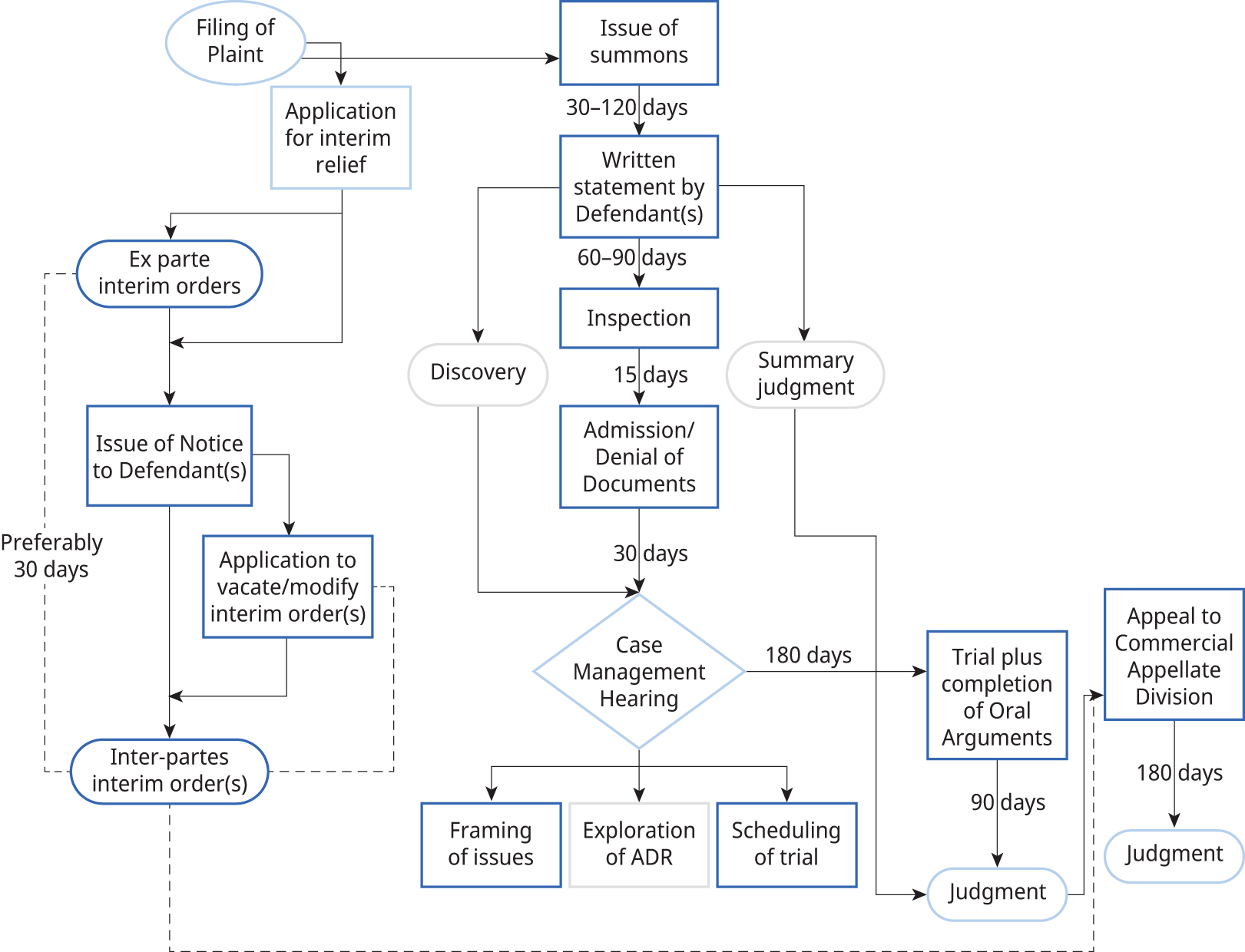
Note: ADR = alternative dispute resolution.
The rigidity of timelines under the Act has been of some concern in patent litigation, given the technical complexity involved. However, most practitioners and litigants agree that, without fixed timelines, litigation tends to become unnecessarily protracted. The strict scheduling ensures that pleadings are completed on time and that trials are expedited. The real bottleneck is the final arguments post-trial, which has systemic causes: chiefly, the enormous number of unfilled positions of judges. Recent trends in filling these vacancies, coupled with specialized training in IP-related matters of judges rostered to IP cases, ought to address the bottleneck problem.
6.6.3.2 Case management hearing
Case management is mandatory under the Commercial Courts Act, 2015. The first case management hearing must be mandatorily held not later than four weeks from the date of filing of an affidavit of admission or denial of documents by the parties. It is intended for the court to engage in the early identification of disputed issues of fact and law, the establishment of a procedural calendar for the entire case (including trial and final hearing), and the exploration of the possibility of dispute resolution other than by trial.
6.6.4 Provisional measures
As a common-law jurisdiction, Indian courts are vested with extensive discretionary powers to grant interim relief. The usual determinants apply: whether the plaintiff has a prima facie case, where the balance of convenience lies, and to whom irreparable injury is likely if the order is or is not granted. An interim order may subject the plaintiff to conditions, including security. Injunctions can be tailored to suit the remedy.137
In general, interim reliefs can be in various forms, including interim injunctions; Mareva orders or freezing orders; Anton Piller orders, where local commissioners (LCs) are appointed with powers of search and seizure; and directions for keeping accounts. Under Order XXXIX(1)–(2) of the Code of Civil Procedure, 1908 [hereinafter the “Code of Civil Procedure”], patentees may also seek interim and ad interim injunctions. Indian courts have regularly considered the grant of interim injunction orders, Anton Piller orders, Mareva orders, Norwich Pharmacal orders or John Doe orders in fitting cases.
It is usual to seek even ex parte ad interim relief in suits for patent infringement. In some cases, where the patent has been tested multiple times in litigation, courts usually even grant the ad interim injunction ex parte; there is no strict rule. For instance, in the case of SEPs, defendants are usually called upon before the grant of an injunction for a response as to whether they are willing to take a license on fair, reasonable and nondiscriminatory terms.
Irrespective of the outcome of the interim proceedings, the parties (usually the unsuccessful party at the interim stage) usually seek an expeditious trial and final hearing. In fact, in one case where the interim injunction was granted in favor of the patentee (i.e., Merck Sharp and Dohme Corp. v. Glenmark Pharmaceuticals),138 the Supreme Court allowed the sale of the existing stock already manufactured by the defendant and directed a day-to-day trial, saying that this was in the national interest, one that demanded a suitable commercial environment for the immediate resolution and adjudication of contentious commercial cases.139 In that case, due to the intervention of the Supreme Court, the time from the suit’s filing to final judgment was only about 30 months. The trial concluded in a record time of less than 30 days. Final arguments were heard for three weeks, and judgment followed very soon thereafter. The Supreme Court has also issued general directions for such expedited hearings in other patent matters.140
In cases where a patent has been tried and tested in prior litigation, courts have not hesitated to grant interim injunctions, though the defendant may be permitted to exhaust existing stocks along with accounts. Some perceive the Delhi High Court to be quite liberal in granting interim injunctions to patentees, though there have been some instances in which the court has refused interim injunctions owing to the complexity of the invalidity defense. In other cases, the court has crafted alternative arrangements for the interim period. Where an interim injunction is refused, courts almost always direct the defendant to maintain and file accounts.
6.6.4.1 Governing legal standards and burdens
Courts see growing numbers of patent litigation, with a corresponding increase in the grant of injunctions (both permanent and interlocutory). Temporary injunctions are regulated by Sections 94 and 95 and Order XXXIX of the Civil Procedure Code. The substantive law on temporary and perpetual injunctions can be found in Sections 36–42 of the Specific Relief Act, 1963.
The general principles for grant or denial of such interim orders are well known: a prima facie case, the balance of convenience, irreparable injury and public interest factors.141 Indian courts have derived principles following the decision of the House of Lords in American Cyanamid v. Ethicon Ltd,142 though the Supreme Court of India has observed that the relatively diluted standard of “prima facie case” in American Cyanamid will not apply in India.143 Similarly, whereas American Cyanamid suggests that more weight must be attached to patents granted after a detailed examination procedure, Section 13(4) of the Patents Act, 1970, and some judicial precedents in India suggest that this proposition is inapplicable to Indian patent law.144
6.6.4.1.1 Prima facie case
The prima facie case requirement is used to discern whether the plaintiff has a reasonable case on merits. It does not finally or conclusively decide issues of fact. It weeds out frivolous or vexatious claims – ones manifestly without merit. As part of this assessment, courts also assess whether defendants have a credible challenge to the suit patent’s validity.145
Initially, in India, a few judicial pronouncements referred to a six-year rule (i.e., a presumption that there could be an increased probability a patent could be treated as valid on the expiry of six years from the date of grant). The genesis of the six-year rule approach can be traced to the Madras High Court’s ruling in Manicka Thevar v. Star Ploro Works case,146 which was subsequently picked up in other judgments.147 However, none of the provisions of law appears to suggest or support this numerical fixation with six years. The Manicka Thevar case was subsequently held not to be correct law by another division bench of the Madras High Court.148 In F Hoffmann-La Roche Ltd v. Cipla Ltd, a single judge of the Delhi High Court also held that there was no basis for the six-year rule and rejected the application of the said rule in patent cases.149 Thus, one will not find a discussion of any such six-year rule in most recent patent cases across India.
6.6.4.1.2 Balance of convenience and public interest
The second requirement for the grant or denial of an interim injunction is that the balance of convenience must be in favor of granting an injunction. The court, while granting or refusing to grant an injunction, should exercise sound judicial discretion to compare and determine the amount of mischief or injury likely to be caused to the respective parties if the injunction is refused and if it is granted. The court would weigh competing possibilities or probabilities.
In India, public interest has been recognized both as a separate factor and as a factor read into the test for the balance of convenience.150 For instance, the public interest in enabling access to lifesaving drugs (both supply and pricing considerations) has been considered a relevant factor when deciding on an application for an interim injunction.151 Recently, given the influence of comorbidity factors such as diabetes and obesity in the severity of COVID-19 infections, the pricing of antidiabetic medications was considered one of the relevant factors when assessing interim injunction applications.152
The defense of public interest is not a complete exception to a legally valid patent and must not be too broadly interpreted, as it would undermine “the rights granted by the sovereign towards monopoly.”153 The Delhi High Court has recognized that upholding the enforcement of patents is also in the public interest.154 Thus, often, public interest factors are considered along with the prima facie strength of the infringement case or the invalidity defense.155
Public interest forms part of the matrix considered by the court and need not always be a dispositive factor in every case. For instance, in Bayer Intellectual Property GmbH v. Ajanta Pharma Ltd,156 factors such as loss of employment and revenue earned by the state – even in cases where the patented drugs were not of a lifesaving nature – were considered by the Delhi High Court. However, in a subsequent decision, Bayer Intellectual Property GmbH v. BDR Pharmaceuticals International Pvt. Ltd,157 another judge of the same High Court held that the export of non-lifesaving drugs would not qualify under the test of public interest merely due to encouraging economic activity and the country earning foreign exchange revenue.
The Delhi High Court, in Merck Sharp and Dohme Corp. v. Glenmark Pharmaceuticals,158 invoked several equitable principles to guide the exercise of discretion in granting injunctions. These included an assessment of the parties’ conduct, whether the defendant attempted to clear the way by filing oppositions or seeking revocation, and so on.
It is also common for defendants to raise pleas of nonworking of patents or nonfiling of working statements to defend against interim injunctions as part of the balance of convenience and public interest factors. The working requirements under the Patents Act, 1970, are stringent. The legislative history shows that the nonworking of patents by foreign companies was one of India’s most significant concerns when drafting the legislation. In a case concerning a patented respiratory disorder drug, the Delhi High Court, in Cipla Ltd v. Novartis AG,159 held that the mere nonmanufacturing of sufficient quantities in India alone could not result in the denial of an interim injunction. However, defendants are free to apply for a compulsory license in such cases. There is an earlier opposing view suggesting that the nonworking of a patent could lead to denial of injunctive relief.160 The later view in Cipla is now the more prevalent view. Though no compulsory license was granted during the COVID-19 pandemic, in dealing with cases relating to shortages of essential medicines used in the treatment of COVID-19, both the Supreme Court and the High Court have made observations favoring such steps being taken by the Government.161
6.6.4.1.3 Irreparable injury
The third and equally important consideration is the condition of irreparable injury. This refers to the patentee having no other remedy available other than an injunction. Irreparable injury, however, does not mean that there must be no physical possibility of repairing the injury. Instead, it means only that the injury must be a material one – namely, one that cannot be adequately compensated in damages.
In Merck Sharp and Dohme Corp. v. Glenmark Pharmaceuticals,162 the Delhi High Court recognized that, in cases where the patentee has been the sole supplier of the patented technology, allowing a defendant to enter the market may cause irreparable injury.
6.6.4.2 Other preliminary reliefs
6.6.4.2.1 Local commissioners
Order XXVI(9) of the Code of Civil Procedure provides for the appointment of LCs. Such LCs are appointed upon the establishment of a strong prima facie case. In cases of a patent infringement action, LCs have been appointed by the Indian courts to record evidence.
An order for the appointment of an LC is usually made in patent litigation if, for example, the manufacturing processes need to be ascertained. The LC is then appointed by the court with strict terms and conditions. Typical conditions imposed include:163
-
that the LC visits the premises of the defendant or plaintiff, as the case may be;
-
that the LC ascertain the manufacturing process being used, including inspection of the raw material registers, excipient data, the quantum of manufacturing and so on;
-
that the accounts of manufacture, sales and so on are inspected;
-
that the LC visits the Customs authorities to retrieve samples of alleged infringing products;
-
permitting the LC to take photographs and videotape the proceedings; and
-
permitting party representatives to accompany the LC, including counsel, to render assistance.
At the end of the execution of the commission, a memorandum of proceedings is prepared by the LC, recording the chronology of events that transpired in the commission and the observations of the LC. This is signed by the LC and the parties, and the LC must give copies of the same to each party. Thereafter, a report is filed before the court, giving a full account of the proceedings. Such a report filed by the LC can be read in evidence without the statement of the LC being recorded in terms of Order XXVI(14)(2) of the Code of Civil Procedure. So long as the court can confirm that the report is genuine and authentic, it forms part of the record. Parties may have objections to the contents of the LC’s report, in which case they can file objections. The objections are then adjudicated by the court before the report is fully read as evidence.
The LC so appointed is not performing a judicial act but a “ministerial act.” Nothing is left to the discretion of the LC, and there is no occasion to use judgment or adjudicate the issues involved. The LC only notes details and reports the actual state of affairs. The LC cannot decide the dispute, but their report helps the court in doing so.164 In short, the LC’s report is one of fact-gathering, not adjudication or determination.
6.6.4.2.2 Interim deposits and other ad interim arrangements
The interim injunction stage may become protracted owing to the technical issues surrounding patents. In such instances, and in a fitting case, courts usually put in an ad interim arrangement. In general, courts enjoy extensive discretion to mold the interim relief to suit the circumstances. For example, courts have directed interim deposits by defendants in SEP cases or have directed the submission of bank guarantees or some form of security to secure the plaintiff’s interest.165 In the recent non-SEP case of Communication Components Antenna Inc. v. Ace Technologies Corp.,166 the High Court’s interim direction to secure the plaintiff by way of a deposit of royalties and bank guarantee was upheld by the Supreme Court of India.167
Equally, in such cases of interim deposits, courts have required the patentee to furnish surety bonds for the amount received on a quarterly basis with advance copies.168
6.6.5 Discovery and gathering of information
At or before the case management hearing, it is usual for parties to admit and deny the respective documents filed by the other party and to also seek discovery, inspection or the production of documents from the other party.169 Upon an appropriate application by one party, the court may direct the other party to respond to written interrogatories on affidavit,170 permit the inspection of documents relied upon or referred by the other party,171 allow discovery of relevant documents on affidavit,172 or direct the production of documents.173
Courts are always empowered to dismiss the suit or defense for want of prosecution if a party does not comply with an order to answer interrogatories or the order for discovery, inspection or the production of documents.174 For instance, in SEP cases, the defendants or the plaintiff would be made to share the claim-mapping charts as part of the exchange of documents to prove that the patents map the standards for which they are sought to be enforced.
Often, discovery and inspection procedures are used to seek irrelevant information and protract litigation. For instance, defendants may seek entire file wrappers from all jurisdictions just as a matter of course. However, courts do not permit such a roving inquiry or fishing expedition, and the party concerned is entitled to move the court to curtail the kind of information or type of documents being sought. The other side of this is “data-swamping” or “data-flooding,” whereby a party against whom disclosure is ordered inundates the other party with all manner of documentation in an attempt to bury the crucial material in a mountain of irrelevance. The Code of Civil Procedure enables parties to seek and defend against any discovery tool, with the courts being the final arbiter if disputes arise in this context. Again, this process demands judicial time (and enough human resources on the bench) and well-honed forensic skills on all sides.
Courts also retain wide discretion in directing the production of documents under certain conditions. To enable discovery and inspection of license agreements, manufacturing processes followed and so on – which may be confidential – courts usually constitute confidentiality clubs to maintain the confidentiality of the information disclosed.175 There has been recent debate as to whether litigants can be part of these confidentiality clubs.176 However, as far as the constitution of the clubs is concerned, there appears to be no dispute; the confidentiality club, once constituted, considerably streamlines the process of discovery and inspection of documents.
Recently, the High Court of Delhi Rules Governing Patent Suits, 2022 have been notified. These rules provide for a minimum mandatory content for pleadings in patent suits,177 a minimum set of mandatory documents to be filed by the parties,178 and specific tweaks in patent suit procedures. For instance, in addition to regular pleadings, the rules provide that parties be allowed to file claim construction, invalidity, infringement briefs and technical primers based on which the court is to frame issues in the first case management hearing.179 A second case management hearing is provided for streamlining the recording of evidence, including the protocol for a hot-tubbing mechanism.180 A reserve third management hearing is provided to address any pending pre-trial concerns.181 Importantly, the rules contemplate the creation of a panel of scientific experts to assist the court.182 While setting the calendar and protocols for a final hearing, the court may also direct that a technical expert of each party may also be present to assist the court.183
6.6.6 Summary proceedings
The Commercial Courts Act, 2015, through its amendment to the Code of Civil Procedure, permits parties to seek summary adjudication.184 Either party may seek such summary disposal if the other party has no real prospect of succeeding and if there is no other compelling reason why the claim should not be disposed of before recording oral evidence.185
Such summary adjudication under Order XIII-A of the Code of Civil Procedure can be sought by filing a specific application and setting out the specific grounds.186 The application is to be filed before issues are framed.187 When adjudicating such an application for summary disposal, courts enjoy broad discretion to pass a variety of orders, including, for instance, conditional orders that require the deposit of money or furnishing security.188
Another possibility of summary disposal is under Order XII(6), by which the court is empowered to pass judgment based on admissions of fact made either in the pleading or even otherwise. The admissions made by patentees during prosecution, whether in India or any foreign jurisdiction, can be construed as admissions under the Code of Civil Procedure and result in summary disposals. The logic is straightforward and self-evident: no patentee should be permitted to make conflicting claims in different jurisdictions. A patentee must be held to be bound by statements made with regard to that specific patent claim, irrespective of where and when that claim is made. This is a species of estoppel.
Such summary procedures help the court to considerably narrow the scope of controversy. For instance, in negotiations for licensing, defendants usually admit that they need the license, and the only dispute that remains is about the licensing amount. In such suits, the court can rely on the correspondence between the parties to issue summary judgments.
Similarly, such summary adjudication has proved workable in SEP litigation: for example, where an ex-licensee of the SEP has refused to renew the license due to a failure of commercial discussions. The dispute is then restricted only to the monetary claim of the licensing fee, and other issues, such as infringement or validity, do not arise. While an ex-licensee is not estopped or precluded from challenging the validity of a patent at any time, courts are reluctant to entertain validity challenges when an erstwhile licensee elects to challenge the patent’s validity only at the time of a license renewal agreement after having enjoyed a license for several years. A court will permit such a challenge to proceed only on a demonstration of some glaring fact that goes to the root of validity and which was noticed at the time of the original licensing.
6.6.7 Evidence
6.6.7.1 Oral evidence and trial
The examination in chief (direct examination) of witnesses is compulsorily on affidavit.189 Cross-examination and reexamination (“redirect”) are taken orally live and transcribed. Often, to save the court’s time, the recording of the oral evidence is done either before the registrar of the court or before an LC. Unlike a court, LCs and registrars are not empowered to rule on objections raised during the evidence.190 However, the commissioner is entitled to enter notes they think material, about a witness’ demeanor so that the same is available to the court at the time of final hearing.191
Usually, trials take between three and five years from the date of filing to conclude, though there have been some patent cases where the trial concluded in six months to a year. Under the Commercial Courts Act, 2015, the court schedules the entire trial so that the recording of evidence is not drawn out. The trial could be day-to-day, and it is common for the courts to explicitly direct as such to reduce inconvenience to witnesses.192 Once the trial of a suit concludes, the matter proceeds to a final hearing.
It is usual for witnesses from foreign jurisdictions to record their statements through videoconferencing. Following the COVID-19 pandemic, virtual courts and online platforms are usually used even for court hearings. Litigants can join proceedings physically, and, if the court has the facility, they can also join the hearing through a videoconferencing facility.
Witnesses are usually in-house representatives or attorneys from the respective parties who have themselves dealt with the litigation and the correspondence between the parties. A witness is not expected to have direct personal knowledge of every part of the deposition; it is enough if the witness can depose to company records and the record of the suit. In some areas, the testimony of people with personal knowledge is preferred – for example, for evidence about discussions in negotiations, the exchange of correspondence, some technical knowledge leading to the grant of the patent and so on.
Other witnesses are usually technical witnesses. In some cases, the inventor is also produced as a witness to strengthen the case of the plaintiff. Experts such as doctors, specialists, economists and accountants have also been produced in the court to establish other aspects of the litigation, such as the calculation of damages, distinguishing the prior art, mapping standards and so on. The inquiry into damages is crucial at the final stage, and, therefore, economists, financial experts or accountants who can analyze and depose to the computation of damages or royalties payable are vitally important in establishing the monetary aspect of the infringement case. Thus, the general practice is to have both in-house and expert witnesses.
6.6.7.2 Who leads evidence first? Can a defendant be directed to lead evidence first?
The Patents Act, 1970, does not specifically provide a procedure for evidence in cases of patent infringement. Instead, the procedure adopted for leading evidence in suits for infringement is in accordance with the Code of Civil Procedure193 and the Indian Evidence Act, 1872. Under the latter, the onus of proof is on the person making a positive assertion. Thus, the patentee-plaintiff must lead evidence first to establish infringement. The defendant leads evidence thereafter to support its defenses or its counterclaim of invalidity. However, this is not a rigid rule. In a case where the defendant admits infringement, and the only question for decision is validity, the court may direct the defendant to lead evidence first. Thus, as provided under Order XVIII, the right to begin is generally granted to the plaintiff:
- 1. Right to begin. – The plaintiff has the right to begin unless the defendant admits the facts alleged by the plaintiff and contends that either in point of law or on some additional facts alleged by the defendant the plaintiff is not entitled to any part of the relief which he seeks, in which case the defendant has the right to begin.
-
2. Statement and production of evidence. –
- (1) On the day fixed for the hearing of the suit or on any other day to which the hearing is adjourned, the party having the right to begin shall state his case and produce his evidence in support of the issues which he is bound to prove.
- (2) The other party shall then state his case and produce his evidence (if any) and may then address the Court generally on the whole case.
- (3) The party beginning may then reply generally on the whole case.
Moreover, where a process claim is asserted, depending on the facts, the burden of proof may shift to the defendant to prove non-infringement. This exceptional situation is provided for under Section 104A of the Patents Act, 1970:
Burden of proof in case of suits concerning infringement.
-
(1) In any suit for infringement of a patent, where the subject matter of patent is a process for obtaining a product, the court may direct the defendant to prove that the process used by him to obtain the product, identical to the product of the patented process, is different from the patented process if, –
- (a) the subject matter of the patent is a process for obtaining a new product; or
-
(b) there is a substantial likelihood that the identical product is made by the process, and the patentee or a person deriving title or interest in the patent from him, has been unable through reasonable efforts to determine the process actually used:
Provided that the patentee or a person deriving title or interest in the patent from him first proves that the product is identical to the product directly obtained by the patented process.
- (2) In considering whether a party has discharged the burden imposed upon him by subsection (1), the court shall not require him to disclose any manufacturing or commercial secrets, if it appears to the court that it would be unreasonable to do so.
Subject to the fulfillment of the condition precedents noted in Section 104A, this is another circumstance in which the defendant may be asked to lead evidence first.194
In Bajaj Auto Ltd v. TVS Motor Co. Ltd,195 the Madras High Court was confronted with a unique situation – a suit against the groundless threat of infringement and non-infringement against the patentee, as well as a subsequent suit for infringement by the patentee. On the limited issue of who should lead evidence first, the court held that the plaintiff in the earlier suit must lead the evidence first since the subsequent suit was more in the nature of a counterclaim of infringement by the patentee. This is yet another unique situation wherein the alleged infringer led evidence first.
6.6.7.3 Filing of affidavits of witnesses in evidence: not treated as evidence till tendered
According to Order XVIII(4) of the Code of Civil Procedure:
Recording of evidence.
-
(1) In every case, the examination-in-chief of a witness shall be on affidavit and copies thereof shall be supplied to the opposite party by the party who calls him for evidence:
Provided that where documents are filed and the parties rely upon the documents, the proof and admissibility of such documents which are filed along with an affidavit shall be subject to the orders of the Court.
- (1A) The affidavits of evidence of all witnesses whose evidence is proposed to be led by a party shall be filed simultaneously by that party at the time directed in the first Case Management Hearing.
- (1B) A party shall not lead additional evidence by the affidavit of any witness (including of a witness who has already filed an affidavit) unless sufficient cause is made out in an application for that purpose and an order, giving reasons, permitting such additional affidavit is passed by the Court.
- (1C) A party shall however have the right to withdraw any of the affidavits so filed at any time prior to commencement of cross-examination of that witness, without any adverse inference being drawn based on such withdrawal: Provided that any other party shall be entitled to tender as evidence and rely upon any admission made in such withdrawn affidavit.
As per Section 1 of the Indian Evidence Act, 1872, affidavits are not included in the ambit of “evidence.” Thus, typically, the affidavit of the witness goes through the process of “tendering” – the witness is put on oath and affirms the contents of the affidavit, and, thus, the affidavit contents effectively become oral evidence. Such oral evidence is normally taken into consideration by the court when facts need to be proved.
6.6.8 Experts
6.6.8.1 Role of experts and expert bodies and institutions
Although not strictly a separate institution, experts and expert bodies and institutions play a key practical role in patent matters. In this context, the Supreme Court of India, in Monsanto Technology LLC v. Nuziveedu Seeds Ltd,196 held that:
Summary adjudication of a technically complex suit requiring expert evidence also, at the stage of injunction in the manner done, was certainly neither desirable or permissible in the law. […]
[…] We are therefore satisfied that the Division Bench ought not to have disposed of the suit in a summary manner by relying on documents only, extracted from the public domain, and not even filed as exhibits in the suit, much less examination of expert witnesses, in the facts of the present case. There is no gain saying that the issues raised were complicated requiring technological and expert evidence with regard to issues of chemical process, biochemical, biotechnical and micro biological processes and more importantly whether the nucleic acid sequence trait once inserted could be removed from that variety or not and whether the patented DNA sequence was a plant or a part of a plant etc. are again all matters which were required to be considered at the final hearing of the suit.
Thus, experts and expert bodies and institutions are a critical component of proceedings where a patent’s validity is questioned. Most oppositions and revocations typically involve one or more opinions from experts or expert bodies, and the legal framework contains sufficient provisions to deal with expert opinions and evidence. For instance, under the Patents Act, 1970, the Indian Patent Office has the power to receive evidence on affidavits, issue commissions for the examination of witnesses or documents and so on.197 The Indian Patent Office may also allow any person to be cross-examined on the contents of their affidavit.198
6.6.8.2 Expert evidence under the Indian Evidence Act, 1872
The Indian Evidence Act, 1872, governs the rules of evidence applicable to enforcement proceedings under the Patents Act, 1970. It applies to all civil and criminal proceedings. This legislation has been amended and updated from time to time, including on the use of electronic documents and evidence.
Section 45 of the Indian Evidence Act, 1872, declares that the opinions of experts are “relevant facts.” Therefore, these opinions must be considered by courts in patent matters when forming an opinion on the point of science or art. The law only requires such experts to be “especially skilled” in the relevant area of science or art without specifying a minimum threshold. The Supreme Court of India has held that an individual could be an expert not just by the special study of the subject but also by acquiring experience in the field.199 Similar is the view of the Delhi High Court which, in a patent case where the expert witness produced did not hold a technology or engineering degree but had proven experience, held that an expert could be a person who possesses experience even if they did not have the educational qualification.200 What is relevant is whether the person is skilled and has adequate knowledge of the subject. The observation of the court reads as follows:
Be that as it may, it is accepted and recognised that a person could be an expert in an area of specialised knowledge by experience and he or she need not hold a degree in the field of specialised knowledge . A person can also become an expert by virtue of one’s avocation or occupation.201
It is generally understood that, in patent matters, the opinions of experts are critical to understanding the background in the art, as well as to appreciating the contents of the prior art and the invention. An expert could also testify as to the meaning of the terms in the claim as understood in the art. Typically, both parties to a patent enforcement action will produce such expert evidence on infringement, novelty and inventive step.
The expert will usually be highly qualified and would exceed the threshold of a person having ordinary skill in the art.
There is a view expressed that the expert in a patent matter must have personal knowledge of the prior arts,202 though this view is not correct. In law, all aspects of patent matters are viewed through the lens of a hypothetical person skilled in the art, who is normally deemed in law to automatically have knowledge of the prior arts. The correct view appears to be that the expert could testify as to their opinion on how a person skilled in the art would consider the matter.
The opinions of such experts are meant for matters of science or art, but, usually, such experts also give their opinions on infringement, novelty, obviousness and other grounds of invalidity. Even though such statements or conclusions on obviousness, novelty or infringement may also involve matters of law, it is not fatal to the admissibility of the expert opinion. Courts will focus more on the reasoning offered by the expert in the opinion. Expert opinions of the experts are not binding on the court.
6.6.8.3 Court-appointed scientific advisers
Section 115 of the Patents Act, 1970, empowers the court to appoint an independent scientific adviser to assist the court or to enquire and report upon any question of fact or opinion (but not involving a question of interpretation of the law). The Indian Patent Office maintains a roster of such scientific experts.203 Courts usually resort to these scientific experts to gain an independent assessment. These assessments are considered valuable in highly contested matters where the parties’ expert testimonies have offered widely disagreeing opinions. Like any other expert opinion, the opinion of a court-appointed scientific adviser is also not binding on the court.
As per Rule 103 of the Patents Rules, 2003, the Controller is to maintain a roll of scientific advisers, to be updated annually. The roll contains the names, addresses, specimen signatures and photographs of scientific advisers; their designations; and information regarding their educational qualifications, the disciplines of their specialization and their technical, practical and research experience.
A person must possess the following qualifications to be enrolled as a scientific adviser:
-
a degree in science, engineering or technology or equivalent;
-
at least 15 years of technical, practical or research experience; and
-
holds or has held a responsible post in a scientific or technical department of the central or state governments or in any organization.204
The law provides that the fee or remuneration for such scientific advisers be provided by the Parliament, by law, for this purpose. However, usually, the parties share the costs of independent scientific experts.
The recently notified draft of the High Court of Delhi Rules Governing Patent Suits, 2020, also proposes the maintenance of a panel of scientific advisers to assist the court.
6.6.8.4 Hot-tubbing procedure
The procedure of hot-tubbing, where multiple expert witnesses give their evidence concurrently – and which has its origin in Australian law – is also permissible in India and has recently been ordered in some cases.205 The procedure for recording expert evidence through a hot-tubbing protocol was specified in Micromax Informatics Ltd v. Telefonaktiebolaget LM Ericsson.206 The Delhi High Court Rules have also been amended to incorporate this procedure,207 including its protocol.208
Though there has yet to be a patent infringement action concluded in which evidence has been given by the hot-tubbing procedure, hot-tubbing is expected to be applied more frequently in the future.
6.6.9 Alternative dispute resolution: pre- and post-litigation mediation
Under the Commercial Courts Act, 2015, parties are usually expected to explore pre-litigation mediation. If the plaintiff does not seek urgent relief, Section 12A of the Commercial Courts Act, 2015, mandates pre-litigation mediation.
Section 89 of the Code of Civil Procedure also recognizes courts’ inherent power to refer parties to arbitration, conciliation, mediation or other forms of alternative dispute resolution. A court can exercise this power at any stage if there exist elements of an acceptable settlement. The parties may also request such a referral themselves.
Almost all district courts and High Courts in India have mediation centers for pre- and post-litigation mediation. These mediation centers are usually attached to each of the High Courts or district courts and are managed by a fully functional secretariat. The mediators at these centers are trained professionals. It is also possible for parties to seek the appointment of an expert mediator with specialized technical learning, skills, experience and domain knowledge. Mediation proceedings have proved to be quite efficient in almost all parts of the country. Significant success has been generally observed in resolving IP rights disputes and, most recently, in the resolution of certain SEP-related disputes.209
6.7 Civil remedies
In a suit for patent infringement, plaintiffs are entitled to seek both interim remedies and final remedies. Section 108 of the Patents Act, 1970, concerns final remedies, whereas Section 94(c) and Order XXXIX of the Code of Civil Procedure concerns interim remedies. The usual types of interim and final remedies – and their governing standards – are discussed in Sections 6.6.4 and 6.7.1–6.7.4 of this chapter, respectively.
6.7.1 Permanent injunction
Under Section 108(1) of the Patents Act, 1970, a patentee may seek a permanent injunction as a final remedy. The Specific Relief Act, 1963, regulates the relief of permanent injunction, and courts retain discretion to deny permanent injunctions in some cases. For instance, in F Hoffmann-La Roche Ltd v. Cipla Ltd,210 a permanent injunction was not granted because the defendant had already been in the market for several years, and the patent was about to expire. Usually, however, permanent injunctions follow a finding of infringement and validity in favor of the patentee.
The court may grant relief by way of an injunction for infringement of a partially valid specification where the invalid claim was framed in good faith and with reasonable skill and knowledge.211 A permanent injunction may be granted only in cases where there is a valid patent, and the defendant has infringed that patent.
6.7.2 Damages or an account of profits
Under Section 108(1) of the Patents Act, 1970, the patentee has the choice of seeking either damages or an account of profits.212 Plaintiffs cannot claim both as per settled law. The Act is silent on the quantification of damages. Unlike the US statute, for instance, the Act does not prescribe a lower threshold of reasonable royalty that has been interpreted to involve the application of the Georgia-Pacific factors.213
The general principle under Indian law is that damages will be compensatory in nature (i.e., the patentee should be restored to the position if the wrongful acts of the defendant had not occurred). Consequently, the measure of the damages is to be, as far as possible, akin to the sum of money that puts the plaintiff in the same position as they would have been in had they not sustained the wrong. Thus, for instance, if the patentee has shown a propensity to license the patent in the past, such licensing arrangements can become the guiding basis in assessing damages.
The reluctance of Indian courts to grant high-value damages is a thing of the past. It is usual, especially in pharmaceutical and SEP cases, for courts to grant damages or accounts of profits determined by the evidence, even if they seem of high value. In a few recent SEP disputes, the royalties payable ran into millions of dollars, even in interim arrangements, though this has not been made known internationally.214 Thus, even in non-SEP cases, damages or accounts of profits are reasonable possibilities, especially if infringement is established by the patentee.
The purpose of an account of profits is to prevent the unjust enrichment of the defendant by the use of the patented invention. The patentee is treated as if they are conducting the business of the defendant and made the profits that the defendant made. As such, the upper limit of an award is the sum of profits made by the patent-infringing defendant. In most cases, an award of damages will equal or exceed the maximum award in an account of profits; however, an account of profits may greatly outstrip an award of damages in the right case.
For an account of profits, the profits must have been earned from the use of the patentee’s invention, and, if the infringed invention formed only part of the overall product or process, then only that part of the profit attributable to the patented invention is recoverable. This is where the most difficulty is experienced in assessing the profits earned by the defendant, and a number of approaches may be taken during the assessment. Courts take the view that it would be unfair to the defendant to award a claim for all the profits where attribution of profits is possible. Where it is appropriate to apportion losses, the reference for the assessment will involve splitting the profits between the infringing and non-infringing parts of the process. Conversely, the patentee could also recover all of the profits of an invention; however, this turns on the facts of the case.
In the event that an infringer makes a loss in a manufacturing process, the sum by which the infringing process reduces those losses are recoverable on account.
Sometimes the patented invention has a readily discernible impact on profits, either positively or negatively. For instance, the patented invention may reduce the costs associated with the manufacturing process, making the process more efficient. In this case, a larger share of the profits would be payable to the patentee on an empirical basis. This would involve a comparison between the profitability achieved when the patented invention was used and when it was not. This brings the efficiencies introduced by the invention into consideration for the calculation of the portion of the profits to be awarded to the patentee.
As per Section 62(2) of the Act, no suit or other proceeding in respect of an infringement of a patent can be instituted during the period between the lapse of the patent (i.e., it had ceased to exist) and the publication of the application restoring the patent.215 A patentee enjoys all entitlements and rights from the date of the patent’s publication.216 However, this right does not extend to instituting any proceedings for infringement until a patent has been validly and finally granted.217 Nevertheless, the claim for damages would also be subject to the laws of limitation.
6.7.2.1 Punitive damages
Along with an account of profits or damages, courts can also impose punitive damages in the following exceptional circumstance: “wrongful conduct by the defendant, which has been calculated by him for himself, which may well exceed the compensation payable to the claimant.”218
The above principle was applied by a single judge of the Delhi High Court recently in Koninklijke Philips NV v. Amazestore,219 which was an SEP case. Indian courts have granted punitive damages in many other cases.220
6.7.2.2 Defenses to avoid damages or an account of profits
Courts refuse a grant of damages or an account of profits if the defendant proves that, at the date of the infringement, they were not aware and had no reasonable grounds for believing that the patent existed.221
The court may also refuse a grant of damages or an account of profits:
-
“in respect of any infringement committed after a failure to pay any renewal fee within the prescribed period and before any extension of that period”;222 or
-
“where an amendment of a specification by way of disclaimer, correction or explanation is allowed under [the Patents Act] after the publication of the specification […] in respect of the use of the invention before the date of the decision allowing the amendment.” However, damages or an account of profits may be granted if “the court is satisfied that the specification as originally published was framed in good faith and with reasonable skill and knowledge.”223
6.7.3 Other remedies
The patentee may further seek the seizure, forfeiture or destruction of infringing articles, as well as of materials and implements predominantly used for the infringing activities.224
6.7.4 Costs
The court may, in its discretion, order the unsuccessful party to pay costs to the successful party in an infringement suit. The Code of Civil Procedure provides for the recovery of costs by and under Sections 35 and 35A.
While imposing costs, the court may weigh several factors: for instance, (i) the conduct of the parties; (ii) whether a party has succeeded only in part, even if that party has not been wholly successful; and (iii) whether the party had made a frivolous claim or counterclaim leading to delay in the disposal of the case, or had instituted a vexatious proceeding wasting the time of the court.
Certain guidelines have been laid down in the case of Ten XC Wireless v. Mobi Antenna225 for determining costs in patent infringement suits, including that
-
the parties shall submit their estimated future cost at the commencement of trial;
-
the parties and court master shall maintain a record of the court time consumed; and
-
the unsuccessful party is liable to pay costs to the successful party.
Costs awarded by the court may include:
-
the actual costs of litigation;226
-
a proportion of another party’s costs;
-
a stated amount in respect of another party’s costs;
-
costs from or until a certain date;
-
costs incurred before proceedings began;
-
costs relating to particular steps taken in the proceedings;
-
costs relating to a distinct part of the proceedings; and
-
interest on costs from or until a certain date.
6.8 Other actions
6.8.1 Cases involving groundless threats of illegal proceedings
6.8.1.1 What constitutes a “threat”?
Keeping in mind the serious negative effects and consequences associated with infringement proceedings, the stated policy of the law is that no person should unnecessarily be subjected to baseless threats of infringement. Under the Patents Act, 1970, groundless threats of infringement are considered civil wrongs.
A “groundless threat” under the Act is an unjustified or wrongful threat by which any person, whether having an interest in the patent or not,227 threatens another with legal proceedings without a reasonable basis. It is important to note that the mere notification of the existence of a patent does not constitute a threat of proceedings within the meaning of the relevant section. In LG Electronics India Pvt. Ltd v. Bharat Bhogilal Patel,228 the Delhi High Court clarified that
if any proprietor or the right holder issues a notice to the custom officials and the custom officials act upon the same by restricting the imports of consignments of any party without the determination (prima facie or otherwise) of the factum of infringement of patent by the appropriate designated authority, then such notice by the right holder to the customs and the actions thereof by the customs either in the form of notice to that party or otherwise calling upon the party to explain its stand are all unnecessary illegal threats to that party.
In Bata India Ltd v. Vitaflex Mauch GmbH,229 even a legal notice was considered a “threat,” and, on facts, it was concluded that threats made by the defendant to the plaintiff were groundless, unjustifiable and wrongful.
6.8.1.2 Remedies
The court typically considers the grant of the following reliefs:
- (a) a declaration to the effect that the threats are unjustifiable;
- (b) an injunction against the continuance of the threats; and
- (c) such damages, if any, as he has sustained thereby.230
The court is also empowered to pass interim orders, as in any other civil suit. For instance, in LG Electronics,231 a suit was initiated on the basis that the filing of a border enforcement action with customs without a finding of infringement from the court amounted to a groundless threat. In the facts of the case, the Delhi High Court passed an interim order staying the operation of a border enforcement action to stop the import of allegedly infringing goods, pending a final decision from a civil court on the issue of infringement.
6.8.2 Declaration of non-infringement
Declaration of non-infringement refers to an application to the court for a declaration that any new process or article does not infringe an existing patent.232Under Section 105 of the Patents Act, 1970, in order to object to declaratory relief, the following conditions precedent need to be fulfilled:
-
The plaintiff has applied in writing to the defendant for a written acknowledgment to the effect of the declaration claimed.
-
The plaintiff has furnished to the defendant the full particulars in writing of its products or process in question.
-
The defendant has refused or neglected to give such an acknowledgment.
Normally, in civil suits, the plaintiff who has sought the relief of non-infringement bears the burden of proof. This was confirmed by the Madras High Court in Bajaj Auto Ltd v. TVS Motor Co. Ltd,233 which went on to hold that, even though the defendant-patentee in the non-infringement filed a counterclaim of infringement, the burden of proof on the person seeking the declaration of non-infringement cannot be reduced or changed.
If the plaintiff in such a declaratory suit is successful, the court can issue a declaratory judgment that the specific product or process of the plaintiff does not infringe the identified patent. At the same time, Section 105(3) of the Patents Act, 1970, stipulates that the court cannot examine the patent’s validity in such proceedings.
6.9 Appellate review
This section is limited to the miscellaneous aspects of review and appeal procedures not otherwise covered in Section 6.2.2. Appeals from orders of the Controller lie to the High Court. Appeals from the orders of a single judge of the High Court usually lie before the division bench of that court or the Supreme Court. Appeals from a commercial court (below the rank of the High Court) lie to the High Court.
Review, in Indian law, is only in case of an error apparent on the face of the record. A party who feels that the forum that rendered the judgment or order committed an error apparent on the face of the record can seek review.
6.9.1 Power of review of the Controller
The Controller has the powers of a civil court in any proceedings filed before it under the Patents Act, 1970, in respect of, among other things, reviewing234 their own decision on an application made235 within one month from the date of communication of such a decision or within an extended period not exceeding one month thereafter as the Controller may allow236 in a prescribed manner.237
6.9.2 Review against civil court orders
Even in civil suits dealing with infringement, review applications may be preferred by parties when the condition for filing a review under Section 114 of the Code of Civil Procedure is fulfilled238. Section 114 of the Code provides as under:
Subject as aforesaid, any person considering himself aggrieved—
- (a) by a decree or order from which an appeal is allowed by this Code, but from which no appeal has been preferred.
- (b) by a decree or order from which no appeal is allowed by this Code, or
-
(c) by a decision on a reference from a Court of Small Causes,
may apply for a review of judgment to the Court which passed the decree or made the order, and the Court may make such order thereon as it thinks fit.
6.9.3 Grounds for review
A review can be filed by any person aggrieved by an order or decree of the Controller or court from which an appeal is allowed, but no appeal has been preferred, or from which no appeal is allowed:
and who, from the discovery of new and important matter or evidence which, after the exercise of due diligence was not within his knowledge or could not be produced by him at the time when the decree was passed or order made, or on account of some mistake or error apparent on the face of the record or for any other sufficient reason, desires to obtain a review of the decree passed or order made against him.239
An application for review must be accompanied by a statement setting forth the grounds on which the review is sought. Where the decision in question concerns any other person in addition to the applicant, the Controller shall forthwith transmit a copy of the application and the statement to the other person concerned.
6.9.4 Appeals from review
No appeal lies from the decision of a Controller rejecting an application for review.240 Even under the Code of Civil Procedure, while an order allowing an application for review is appealable, an order rejecting an application for review is not.241
6.10 Selected topics
6.10.1 Compulsory licenses and public prejudice
Compulsory license is not directly relevant to the revocation of a patent. However, unlike voluntary licensing, it is a form of licensing that is involuntary and coercive in nature. Consistent with Article 31 of the TRIPS Agreement and the Doha Declaration, where there is a failure to fulfill the reasonable demand for the patented invention, or in a case of unreasonable pricing or failure to work the invention on a commercial scale, an interested person can seek a compulsory license.242 The conditions for invoking compulsory licensing provisions are strict and must be fulfilled before such a license can be issued. Thus, there are hardly any compulsory licenses issued in India. The rare occasion when such a license was issued is a case of a cancer drug in which the courts came to the conclusion that the requirements of the public were not being met.243
Such compulsory licenses, if issued, would ordinarily only be for the domestic market and, among other stipulations, upon payment of a reasonable royalty.244 Pursuant to Article 31bis of the TRIPS Agreement, a compulsory license can also be granted for the export of pharmaceutical products to countries with insufficient or no manufacturing capacity for that product. Although it is directed toward the greater public interest, compulsory license proceedings are adversarial in nature, and a detailed procedure complying with natural justice has been prescribed,245 as have appeals from such decisions.246 Under Section 92(3), the Central Government may notify the existence of a national emergency, extreme emergency or a case of public noncommercial use, and the detailed procedure would stand suspended.
However, in the unique and exceptional situation where the underlying cause resulting in the compulsory license is not addressed even after two years from the date of granting the compulsory license, the Central Government may, for any interested person, apply to the Controller to revoke the patent.247 Interestingly, the Justice N Rajagopala Ayyangar Committee had proposed this provision on the logic that the threat of revocation was a sufficient incentive for the patentee to share any know-how associated with the working of the invention. This relation between the working of the invention and the sharing of associated know-how has been raised in the context of COVID-19 vaccination.248
Similarly, the Patents Act, 1970, also empowers the Central Government to use the invention for the purposes of the Government,249 and, if the patentee refuses to comply with the Government’s request and on unreasonable terms, the Central Government is authorized to seek revocation of the patent before the High Court.250
Independently, Section 66 of the Act reserves to the Central Government the residual power to declare a patent as revoked if the patent or the mode in which it is exercised is mischievous to the state or generally prejudicial to the public. This provision requires an opportunity for hearing being granted to the patentee before any such declaration or decision.
6.11 Key challenges and efforts to improve patent case management
6.11.1 Lack of uniformity in decisions and specialized knowledge
Infringement cases are heard by judges of either the district courts or the High Courts not specially trained in the subject of patent law. A challenge that arises under the current regime of trial, especially before the district courts, is that the judges are not equipped to understand technical issues that arise in respect of patents. As the disputes arising under the Patents Act, 1970, are often highly technical in nature, a higher degree of understanding of the subject matter is required to ascertain a question of infringement or lack thereof.251 For this purpose, specific rules of procedure, including for leading and examination of evidence, are desirable.
6.11.2 Delays in disposing of suits
The Supreme Court has held, pertaining to the issue of delays in the hearing and disposal of matters, that, in matters of patents, trademarks and copyrights, the state of affairs due to such delays is unsatisfactory.252 It was held that there is a need to adhere to the provisions of Order XVII(1)(2) of the Code of Civil Procedure, whereby proceedings are to be held on a daily basis for quick disposal of the suit. The streamlined and expedited procedure under the Commercial Courts Act, 2015, has significantly helped in reducing delays, though more groundwork is needed. A recent development which has given further impetus to speedier adjudication of IP disputes is the establishment of the Intellectual Property Division in the Delhi High Court.
6.11.3 The IP Division, Delhi High Court
Post the abolition of the IPAB,253 all pending cases before the IPAB were transferred to High Courts. The High Courts were thus faced with the task of managing both the already pending IPR cases before them as well as the transferred IPAB cases. In the Delhi High Court, this number of pending IP cases was expected to be around 4,000–5,000. With a view to streamlining dispute resolution of IP cases, including patent litigation, the Delhi High Court constituted India’s first “IP Division,”254 The Division uses specialized sets of Rules, specifically, the Delhi High Court Intellectual Property Rights Division Rules, 2022255 and the High Court of Delhi Rules Governing Patent Suits, 2022. The Division is vested with various jurisdictions, including the original jurisdiction, the infringement jurisdiction, commercial suits, the appellate jurisdiction from the IP offices, the revisional jurisdiction from the commercial courts, and the extraordinary writ jurisdiction supervising all IP offices, including the Controller of Patents’ Office.256 Further, the Division provides for various novel features which are, illustratively, as under:
- (i) New forms of evidence recording such as hot-tubbing and remote recording of evidence.257
- (ii) Preservation of evidence and litigation hold notice.258
- (iii) Guidance on computation of damages.259
- (iv) Direction of consolidation of cases.260
- (v) Constitution of Confidentiality Clubs and redaction of information.261
- (vi) Summary adjudication of disputes.262
- (vii) Panel of experts and recruitment of law researchers who are technically qualified like engineers, chemists, pharmacists, or civil engineers, to assist the IP Division.263
- (viii) Mediation and Early Neutral Evaluation.264
While the creation of the Division on a non-exclusive basis was notified in July, 2021,265 the Rules for the Division in its current form, that is the Delhi High Court Intellectual Property Rights Division Rules, 2022, vesting exclusive jurisdiction in the IP Division and laying down procedure for IP disputes, were notified in February 2022. This Division now consists of three Single Judge Benches exclusively dealing with IPR disputes. Since the setting up of the Division, the disposal rate for IP cases, specifically in patent matters, has witnessed substantial improvement. In addition the Delhi High Court has also nominated a non-exclusive IP Appellate Division consisting of a Bench of two Judges which hears appeals from the IP Division.
As of July 2022, the IP Division of the Delhi High Court has approximately 4,000 pending IP disputes out of which a substantial number are trademark and copyright disputes. Patent cases account for approximately 600–650 cases, most of which are appeals transferred from the IPAB. Notably, the filing of patent infringement actions has risen in recent times and approximately 50–60 actions are filed annually before the Court. In the IP Division, trends show that the disposal figures are rising and it is estimated that the Division should bring in greater efficiency.
The IP Division of the Delhi High Court has also been received well internationally. The recent USTR 2022 Special 301 Report discusses the establishment of the Delhi High Court’s IP Division as a positive development and emphasizes continued engagement of the U.S. with India on IP matters.266
As far as other states are concerned, in the Bombay High Court and the Madras High Court, post the abolition of the IPAB, the total number of pending patent disputes is, approximately, around 450–500 cases and 264 cases respectively. The Gujarat High Court has in fact nominated a non-exclusive bench for adjudication of all IP disputes.
On this note, a testament to the Delhi High Court IP Division’s efficacy in streamlining dispute resolution of IP cases may also be found in the fact that the Parliamentary Committee which had been set up to look into IP issues in India had earlier recommended (in 2021), to re-establish the IPAB, stating that the abolition decision was one taken in a hurry and there should have been stakeholder consultation.267 However, post-establishment of the IP Division in the Delhi High Court, in April 2022 the Committee revised its recommendation268 and recommended that IP divisions like at the Delhi High Court should be established in all High Courts in the country. The relevant observations of the Report dated April 6, 2022 read:
3.12 The Committee notes that the dissolution of IPAB would lead to transferring of all IP-related appeals including the pending cases to High Courts and Commercial Courts (in copyright matters). This may create additional burden on such courts which are already reeling under huge backlog of cases with inadequate expertise in hand to deal with IPR matters. It, therefore, opines that establishing an Intellectual Property Division (IPD) with dedicated IP benches as done by Delhi High Court in the wake of abolition of IPAB would ensure effective resolution of IPR cases on a timely basis. The Committee, therefore, recommends that the Government should take appropriate measures to encourage setting up of IPD in High Courts for providing alternative solution to resolve IPR cases.
Thus, the constitution of specialized IP Divisions for speedier and efficacious resolution of IP disputes, specifically patent litigation, seems to be the way forward for India.
AIR 2013 SC 1311.
E.g., Merck Sharp and Dohme Corp. v. Glenmark Pharmaceuticals, 2015 SCC Online Del. 8227; Cipla Ltd v. Novartis AG, 2017 SCC Online Del. 7393; Symed Labs v. Glenmark Pharma Ltd, 2015 SCC Online Del. 6745.
E.g., UPL Ltd v. Pradeep Sharma, 2018 SCC Online Del. 7315.
E.g., Koninklijke Philips NV v. Amazestore, 260 (2019) DLT 135; Telefonaktiebolaget LM Ericsson (Publ.) v. Intex Technologies (India) Ltd, 2015 SCC Online Del. 8229 (a final decree concerning SEPs); Koninklijke Philips NV v. Vivo Mobile Communications Co. Ltd, CS (COMM) 383 of 2020; Koninklijke Philips NV v. Xiaomi Inc., CS (COMM) 502 of 2020.
E.g., Shogun Organics Ltd v. Gaur Hari Guchhait, 263 (2019) DLT 516; Eisai Co. Ltd v. Satish Reddy, 2019 SCC Online Del. 8496.
E.g., F Hoffmann-La Roche Ltd v. Cipla Ltd, MIPR 2016 (1) 1; Koninklijke Philips, 260 DLT; Shogun Organics, 263 DLT (damages were awarded in the sum of about USD 25 million).
E.g., AstraZeneca AB v. Intas Pharmaceuticals Ltd, MANU/DE/1939/2020; B Braun Melsungen AG v. Rishi Baid, MANU/DE/0376/2009; Arif Abdul Kader Fazlani v. Hitesh Raojibhai Patel and Co., MANU/GJ/1304/2011; F Hoffmann-La Roche Ltd v. Cipla, 159 (2009) DLT 243 (DB).
AIR 1982 SC 1444, para. 17.
General Agreement on Tariffs and Trade, Oct. 30, 1947, 55 UNTS 194.
Panel Report, India – Patent Protection for Pharmaceutical and Agricultural Chemical Products, WTO Doc. WT/DS50/R (Sep. 5, 1997).
Appellate Body Report, India – Patent Protection for Pharmaceutical and Agricultural Chemical Products, WTO Doc. WT/DS50/AB/R (Dec. 19, 1997).
Panel Report, India – Patent Protection for Pharmaceutical and Agricultural Chemical Products, WTO Doc. WT/DS79/R.
WTO, Ministerial Declaration of 14 November 2001, WTO Doc. WT/MIN(01)/DEC/1, 41 ILM 746 (2002), para. 5 (“Accordingly, and in the light of paragraph 4 above, while maintaining our commitments in the TRIPS Agreement, we recognize that these flexibilities include: […] (b). Each Member has the right to grant compulsory licences and the freedom to determine the grounds upon which such licences are granted”).
WTO, Ministerial Declaration of 14 November 2001, WTO Doc. WT/MIN(01)/DEC/1, 41 ILM 746 (2002), para. 6.
Patents Act, 1970, §24B(1)(b).
Patents Act, 1970, §24B(1).
Patents Act, 1970, §24A(1).
Patents Act, 1970, §24A(2).
Patents Act, 1970, §24A(3).
Patents Act, 1970, §24D.
Patents Act, 1970, §60.
Patents Act, 1970, §2(1)(j).
Patents Act, 1970, §2(1)(ja).
Patents Act, 1970, §3.
Patents Act, 1970, §11-A.
Patents Act, 1970, §104-A.
Patents Act, 1970, ch. VI.
Patents Act, 1970, §86.
Patents Act, 1970, §107A(b).
Patents Act, 1970, ch. XIX.
The Tribunals Reforms (Rationalisation and Conditions of Service) Ordinance, 2021 (April 4, 2021).
Patents Act, 1970, §157-A.
The Tribunals Reforms (Rationalisation and Conditions of Service) Ordinance, 2021 (April 4, 2021).
These patent offices are the European Patent Office, United State Patent and Trademark Office, Japan Patent Office, United Kingdom Intellectual Property Office, Canadian Intellectual Property Office, German Patent and Trade Mark Office, Intellectual Property Australia, India Office of the Controller General of Patents, Designs and Trade Marks, National Institute of Industrial Property of Chile, Intellectual Property Corporation of Malaysia, Russian Federal Service for Intellectual Property, Peru National Institute for the Defense of Competition and Protection of Intellectual Property and Spanish Patent and Trademark Office.
See Discussion on the Trade Marks Bill, 1999, Lok Sabha Debates, Session No. 2, Dec. 22, 1999, 454, at 455 (“[The Trade Marks Bill, 1999] seeks to provide for an Appellate Board for the speedy disposal of appeals and rectification of application which presently before the High Court”).
The Tribunals Reforms (Rationalisation and Conditions of Service) Ordinance, 2021.
Pfizer Products Inc. v. Controller of Patents and Designs, 2020 SCC Online IPAB 19; Dhaval Diyora v. Union of India, 2020 SCC Online Bom. 2550; Anaghaya Million Pharma LLP v. Nippon Soda Co. Ltd, MANU/IC/0074/2020.
(2012) 13 SCC 429, at 432. The Court held: “considering the fact that the Report of the Opposition Board can be crucial in the decision making process, while passing order by the Controller under Section 25(4), principles of natural justice must be read into those provisions. Copy of the Report/recommendation of Opposition Board, therefore, should be made available to the parties before the Controller passes orders under Section 25(4) of the Act.”
Aloys Wobben v. Enercon, 2010 SCC Online Mad. 4668.
Sankalp Rehabilitation Trust v. F Hoffmann La-Roche, 2012 SCC Online IPAB 167.
UCB Farchim v. Cipla Ltd, 2010 SCC Online Del. 523.
Such High Courts are those of Allahabad, Chennai, Mumbai, Calcutta, Punjab and Haryana, Delhi, Madhya Pradesh, Patna, and Jammu and Kashmir.
Until 2021, this was the IPAB, Following the Tribunals Reforms (Rationalisation and Conditions of Service) Ordinance, 2021, this jurisdiction is vested with the High Court.
2012 SCC Online Del. 2259.
Aloys Wobben v. Yogesh Mehra, (2014) 15 SCC 360.
Bishwanath Prasad v. Hindustan Metal Industries, (1979) 2 SCC 511. But see Farbwerke Hoechst v. Unichem Laboratories, AIR 1969 Bom. 255 (holding that specification must be referred to only in the case of ambiguity. However, the Supreme Court’s judgment in Bishwanath Prasad v. Hindustan Metal Industries effectively overrules this judgment).
MIPR 2016 (1) 1.
(1982) RPC 183.
[1982] RPC at 244.
Improver Corp. v. Remington Consumer Products Ltd, [1990] FSR 181.
Kirin-Amgen Inc. v. Hoechst Marion Roussel Ltd, [2005] RPC 9.
[2017] UKSC 48.
[2019] FSR 5.
H Lundbeck A/S v. Hetro Drugs Ltd, CS (COMM) 565 of 2020, order dated Dec. 23, 2020.
F Hoffmann-La Roche Ltd v. Cipla Ltd, MIPR 2016 (1) 1.
Indian Patents and Designs Act, 1911, §29(1).
Raj Parkash v. Mangat Ram Chowdhry, 1977 SCC Online Del. 33, para. 25.
Parkash, 1977 SCC Online Del. 33, para. 25.
CTR Manufacturing Industries Ltd v. Sergi Transformer Explosion Prevention Technologies Pvt. Ltd, 2015 SCC Online Bom. 5538; Novartis AG v. Adarsh Pharma, 2004 SCC Online Mad. 402.
WP (C) 1971 of 2014, order dated March 8, 2017, appealed in 2019 SCC Online Del. 8209.
Kapil Wadhwa v. Samsung Electronics Co. Ltd, 2012 SCC Online Del. 5172, appeal filed, Samsung Electronics Co. Ltd v. Kapil Wadhwa, CA 8600 of 2013.
Warner Bros. Entertainment Inc. v. Santosh VG, CS (OS) 1682 of 2006, order dated April 13, 2009; Engineering Analysis Centre of Excellence Pvt. Ltd v. Commissioner of Income Tax, 2021 SCC Online SC 159.
F Hoffmann-La Roche Ltd v. Cipla Ltd, MIPR 2016 (1) 1.
(2013) 6 SCC 1.
[2008] EWCA Civ 192; see also Richard Miller et al., Terrell on the Law of Patents, para. 9.14 (18th ed. 2016).
AstraZeneca AB v. P Kumar, 262 (2019) DLT 118; AstraZeneca AB v. Intas Pharmaceuticals, I.A. 8826/2020 in CS (COMM) 410 of 2020, order dated Nov. 2, 2020. But see AstraZeneca AB v. Zydus Healthcare, CS (COMM) 414 of 2020, order dated Nov. 18, 2020. The 2020 orders are presently under appeal before a division bench of the Delhi High Court.
Indian Evidence Act, 1872, §101.
Commercial Courts Act, 2015, §2(1)(c)(vii).
2019 (12) SCC 210.
2015 SCC Online Del. 8227.
Glenmark Pharmaceuticals Ltd v. Merck Sharp and Dohme Corp., (2015) 6 SCC 807.
Bajaj Auto v. TVS Motors, (2009) 9 SCC 797; Az Tech (India) v. Intex Technologies (India), SLP (C) 18892 of 2017, order dated Aug. 16, 2017.
F Hoffman-La Roche Ltd v. Cipla Ltd, 2009 (110) DRJ 452 (DB); Merck Sharp and Dohme Corp. v. Glenmark Pharmaceuticals, 2015 SCC Online Del. 8227.
[1975] AC 396.
SM Dyechem Ltd v. Cadbury (India) Ltd, (2000) 5 SCC 573.
F Hoffman-La Roche, (110) DRJ, paras 53–55 (DB).
F Hoffman-La Roche, (110) DRJ, paras 53–55 (DB).
AIR 1965 Mad. 327.
National Research and Development Corp.’s, Bilcare v. Amartara Pvt. Ltd., 2007 (34) PTC 419 (Del).
Mariappan v. AR Safiuallah, 2008 (5) CTC 97.
2008 (37) PTC 71 (Del.) (SJ), aff’d, 159 (2009) DLT 243 (DB) (though there is no express discussion on the six-year rule).
F Hoffmann-La Roche, 159 DLT (DB).
F Hoffmann-La Roche, 159 DLT (DB).
AstraZeneca AB v. Intas Pharmaceuticals Ltd, MANU/DE/1939/2020.
Novartis AG v. Cipla Ltd, 2015 SCC Online Del. 6430, para 88.
Merck Sharp and Dohme Corp. v. Glenmark Pharmaceuticals, 2015 SCC Online Del. 8227.
Merck Sharp and Dohme, 2015 SCC Online Del.; see also Bristol-Myers Squibb Co. v. JD Joshi, CS (OS) 2303 of 2009; Bristol-Myers Squibb Co. v. D Shah, CS (OS) 679 of 2013.
CS (COMM) 1648 of 2016, order dated Jan. 4, 2017.
CS (COMM) 107 of 2017, order dated Feb. 16, 2017.
Merck Sharp and Dohme, 2015 SCC Online Del.
2017 SCC Online Del. 7393.
See Franz Xaver Humer v. New Yash Engineers, (1996) ILR 2 Del. 791.
In re Distribution of Essential Supplies and Services during Pandemic, Suo Motu Writ Petition (Civil) 3 of 2021, order dated April 30, 2021; Rakesh Malhotra v. Government of National Capital Territory of Delhi, WP (C) 3031 of 2020, order dated April 20, 2021.
Merck Sharp and Dohme, 2015 SCC Online Del. 8227.
E.g., Victoria Foods Private Limited v. Rajdhani Masala Co. & Anr., CS(COMM) 108 of 2021, order dated March 24, 2022; Sun Pharma Laboratories Ld. v. Interio International P. Ltd & Ors., CS(COMM) 184 of 2022, order dated March 28, 2022.
Saraswathy v. Viswanathan, 2002 (2) CTC 199.
E.g., Telefonaktiebolaget LM Ericsson (Publ.) v. Intex Technologies (India) Ltd, CS (OS) 1045 of 2014, judgment and order dated March 13, 2015; Dolby International v. GDN Enterprises Pvt. Ltd, CS (COMM) 1425 of 2016, orders dated Oct. 27, 2016, and Nov. 23, 2016; Koninklijke Philips NV v. Vivo Mobile Communications Co. Ltd, CS (COMM) 383 of 2020; Koninklijke Philips NV v. Xiaomi Inc., CS (COMM) 502 of 2020.
CS (COMM) 1222 of 2018, order dated July 12, 2019.
Communication Components Antenna Inc. v. Ace Technologies Corp., SLP (C) 21938 of 2019, order dated Sep. 20, 2019.
Telefonaktiebolaget LM Ericsson v. Mercury Electronics, 2015 (64) PTC 105 (DEL).
M Sivasamy v. Vestergaard Frandsen A/S, 2009 (113) DRJ 820 (DB); Telefonaktiebolaget LM Ericsson (Publ.) v. Lava International Ltd, CS (OS) 764 of 2015, order dated March 1, 2016; Pfizer Inc v. Union Remedies Ltd, 2016 SCC Online Bom. 8599; Delhi High Court (Original Side) Rules 2018, ch. VII r. 17.
Interdigital Technology v. Xiaomi Corp., CS (COMM) 295 of 2019, order dated Oct. 9, 2020.
Monsanto Technology LLC v. Nuziveedu Seeds Ltd, (2019) 3 SCC 381.
2010 SCC Online Mad. 5031.
(2019) 3 SCC 381, paras 22–23.
State of Himachal Pradesh v. Jai Lal, (1999) 7 SCC 280.
Vringo Infrastructure Inc. v. ZTE Corp., FAO (OS) 369 of 2014, order dated Aug. 13, 2014.
Vringo Infrastructure, FAO (OS) at para. 11.
F Hoffmann-La Roche Ltd Cipla Ltd, MIPR 2016 (1) 1.
E.g., Micromax Informatics Ltd v. Telefonaktiebolaget LM Ericsson, MANU/DE/1477/2019; Telefonaktiebolaget LM Ericsson (Publ.) v. Intex Technologies (India) Ltd, CS (COMM) 769 of 2016, order dated Jan. 30, 2019.
MANU/DE/1477/2019.
Delhi High Court (Original Side) Rules 2018, ch. XI r. 6.
Delhi High Court (Original Side) Rules 2018, annex G.
Justice Prathiba M. Singh, “Samadhan-Mediation in Delhi” in National Conference on Mediation and Information Technology (High Court of Gujarat ed., 2022). “In Intellectual property rights (“IPR”) disputes however, the average percentage of settlements arrived at is a whopping 84.50%…Notably, 2017 was a rare year for the [Delhi High Court Mediation and Conciliation] Centre which saw 100% of all IPR matters referred, being settled. Even thereafter the percentages of settlement in IPR matters are hovering around 85% to 95% in the pre-pandemic years. Compared to IPR matters, the percentage of commercial disputes that are settled is comparatively lesser…”
MIPR 2016 (1) 1.
Georgia-Pacific Corp. v. United States Plywood Corp., 318 F. Supp. 1116 (S.D.N.Y. 1970), mod. and aff’d, 446 F. 2d 295 (2d Cir. 1971), cert. denied, 404 U.S. 870 (1971).
See Telefonaktiebolaget LM Ericsson v. Mercury Electronics, 2015 (64) PTC 105 (DEL).
In G Srinivasan v. Voltamp Transformers, AIR 2017 Mad. 144, and Koninklijke Philips Electronics NV v. Rajesh Bansal, 251 (2018) DLT 602, the courts refused damages for the period after the expiry of the patent.
Hindustan Unilever Ltd v. Reckitt Benckiser India Ltd, (2014) ILR 2 Del. 1288, para 66 (citing Rookes v. Barnard, [1964] AC 1129).
260 (2019) DLT 135.
See Vior (International) Ltd v. Maxycon Health Care Pvt. Ltd, 2018 (74) PTC 87 (Del.).
2011 SCC Online Del. 4648.
E.g., Merck Sharp and Dohme Corp. v. Glenmark Pharmaceuticals Ltd, (2015) 6 SCC 807 (the plaintiffs were granted actual costs of the entire litigation proceedings); Austin Nichols and Co. v. Arvind Behl, 2005 SCC Online Del. 1276 (the Delhi High Court awarded INR 1,885,000 in favor of the plaintiff). No general or statutory rules apply; the same is at the discretion of the court.
2012 (51) PTC 513 (Del.), para 97.
222 (2015) DLT 498.
(51) PTC.
2010 SCC Online Mad. 5031.
Grounds for review could be “a mistake” or “error apparent on the face of the record” “or any analogous ground” or “to prevent miscarriage of justice”. See Shivdev Singh & Ors. v. State of Punjab & Ors., AIR 1963 SC 1909.
Bayer v. Union of India, AIR 2014 Bom. 178.
In Re: Distribution of Essential Supplies and Services during the Pandemic, Suo Motu Writ Petition (Civil) No.3 of 2021, Order dated April 30, 2021; Dharmendra Kumar Aggarwal v. GNCTD, W.P.(C) 5173/2021, Order dated May 5, 2021.
Onyx Therapeutics Inc. v. Union of India, 2019 SCC Online Del. 11881.
Bajaj Auto Ltd v. TVS Motor Co. Ltd, 2009 (3) ARC 414.
Ministry of Commerce and Industry (IPR-Estt. Section), Government of India, Notification S.O.1668(E), April, 22, 2021.
High Court of Delhi, Office Order No.667/Original Side/DHC, July, 7, 2021.
High Court of Delhi, Office Order No.667/Original Side/DHC, July 7, 2021.
Office of the United States Trade Representative, 2022 Special 301 Report, April 2022, page 56.
Department Related Parliamentary Standing Committee on Commerce (Rajya Sabha, Parliament of India), Review of the Intellectual Property Rights Regime in India, Report No.161, July, 2021/ Shravana, 1943 (Saka), July 23, 2021, paras. 9.7–9.8.
Department Related Parliamentary Standing Committee on Commerce (Rajya Sabha, Parliament of India), Action Taken by Government on the Recommendations/ Observations of the Committee contained in its One Hundred and Sixty First Report on “Review of the Intellectual Property Rights Regime in India,” Report No. 169, April, 2022/ Chaitra 1944 (Saka), April 6, 2022, para. 3.12.