PCT International Search and Preliminary Examination Guidelines
PART III EXAMINER CONSIDERATIONS COMMON TO BOTH THE INTERNATIONAL SEARCHING AUTHORITY AND THE INTERNATIONAL PRELIMINARY EXAMINING AUTHORITY
Chapter 10 Unity of Invention
Alternative Forms of an Aspect of the Invention (Varying Solutions to the Same Problem)
Unity of Invention Exists - Examples 24 to 30
10.44 Example 24
Claim 1: A chair with a lifting mechanism.
Claim 2: A chair with a mechanical screw lifting mechanism.
Claim 3: A chair with a hydraulic lifting mechanism.
Unity exists a priori between claims 1, 2 and 3 because the special technical feature common to all the claims is a chair with a lifting mechanism. However, if a chair with a lifting mechanism is known in the art, there would not be a special technical feature common to all the claims and unity of invention would be lacking.
10.45 Example 25(A) (Common Structure)
Claim 1: A compound of the formula:

wherein R1 is selected from the group consisting of phenyl, pyridyl, thiazolyl, triazinyl, alkylthio, alkoxy, and methyl; R2-R4are hydroxyl, methyl, benzyl, or phenyl.
The compounds are useful as pharmaceuticals for the purpose of enhancing the capacity of the blood to absorb oxygen.
In this case, the indolyl moiety is the significant structural element that is shared by all of the alternatives. Since all the claimed compounds are alleged to possess the same utility, unity is present. This is consistent with "Markush practice" wherein a special technical feature is provided by a commonly shared structure which constitutes a structurally distinct portion in view of the prior art and the common structure is essential to the common property or activity (see paragraph 10.17).
Example 25(B) (Common Structure including a proviso in claim 1)
Claim 1: A compound of the formula:

wherein R1 is selected from the group consisting of phenyl, pyridyl, thiazolyl, triazinly, alkylthio, alkoxy, and methyl; R2-R4 are hydroxyl, methyl, benzyl, or phenyl with the proviso that R2 and R3 cannot both be methyl.
The compounds are useful as pharmaceuticals for the purpose of enhancing the capacity of the blood to absorb oxygen.
In this case, the indolyl moiety is the significant structural element that is shared by all of the alternatives. Since all the claimed compounds are alleged to possess the same utility, unity is present a priori. However, prior art that shows compounds having the same use and having this shared core structure could be used to show that this claim lacks unity of invention a posteriori. This prior art could even include prior art where both R2 and R3 are methyl since the prior art only needs to teach what is shared (i.e., the prior art does not need to anticipate/render obvious the claims).
Example 25(C) (Common Structure including a functional limitation in claim 1)
Claim 1: A compound of the formula (I) having the property of enhancing the capacity of the blood to absorb oxygen:
 Formula (I)
Formula (I)
wherein R1 is selected from the group consisting of phenyl, pyridyl, thiazolyl, triazinly, alkylthio, alkoxy, and methyl; R2-R4 are hydroxyl, methyl, benzyl, or phenyl.
In this case, the indolyl moiety is the significant structural element that is shared by all of the alternatives. Further, the specification and claims teach that all of the compounds of Formula (I) possess the claimed utility. Thus, unity is present a priori. Prior art teaching the shared structures possessing the claimed function could be used to show that this claim lacks unity of invention a posteriori.
For some Authorities, even for prior art which teaches Formula (I) wherein R1 is phenyl and R2-R4 are methyl, but is silent with regard to the claimed function, the lack of unity would be applicable since it would be regarded as inherently possessing the claimed function as evidenced by applicants' specification and/or other reference(s) which evidence this point, whether that evidence was published before or after Applicants' filing date since a compound and its properties are inseparable.
Example 25(D) (Common Structure including a functional alternative in claim 2)
Claim 1: A compound of the formula (I):
 Formula (I)
Formula (I)
wherein R1 is selected from the group consisting of phenyl, pyridyl, thiazolyl, triazinly, alkylthio, alkoxy, and methyl; R2-R4 are hydroxyl, methyl, benzyl, or phenyl.
Claim 2: The compound of claim 1 where R1 is pyridyl and R2-R4 are methyl.
The compounds are useful as pharmaceuticals for the purpose of enhancing the capacity of the blood to absorb oxygen.
In this case, the indolyl moiety is the significant structural element that is shared by all of the alternatives. Further, the specification and claims teach that all of the compounds of Formula (I) possess the claimed utility. Thus, unity is present a priori.
If unity of invention is lacking a posteriori then some Authorities would determine that the first named invention searched would be a compound of Formula (I) wherein R1 is phenyl (not pyridyl) and R2-R4 are hydroxyl (not methyl) since those are the first named Markush members listed in claim 1 (see also Example 34). Note in such cases that the first claimed invention does not include any sub-genuses, but rather is limited to the first embodiment for each variable. However, the Authority/examiner may at their discretion and depending on the specific circumstances of the case include one or more sub-genuses within the first invention.
Alternatively, an Authority may determine that the first invention searched may comprise different groupings. In most cases this determination will be made based on a consideration of the circumstances of the case including the interdependence of the different groups, the specific examples given in the application and the prior art that has been identified. For example, in the above case it may be considered that the phenyl, pyridyl, thiazolyl and triazinyl groups share a common property in that they are aromatic rings. However, prior art disclosing compounds having the same activity and comprising an aromatic group such as pyrimidine in this position could be used to raise a lack of unity between each of these groups.
In a further alternative, some Authorities may determine that each type of the first substituent, here R1, is as the special technical feature; in other words if the Formula (I) wherein R1 is phenyl is known from the prior art, then the first invention is the Formula (I) wherein R1 is pyridyl and R2-R4 are hydroxyl, methyl, benzyl or phenyl (i.e. all options for R2-R4); the second invention is the formula wherein R1 is thiazolyl and R2-R4 are hydroxyl, methyl, benzyl or phenyl, and so on.
10.46 Example 26 (Common Structure)
Claim 1: A compound of the formula:

wherein R1 is selected from the group consisting of phenyl, pyridyl, thiazolyl, triazinyl, alkylthio, alkoxy, and methyl; Z is selected from the group consisting of oxygen (O), sulfur (S), imino (NH), and methylene (-CH2-).
The compounds are alleged to be useful as pharmaceuticals for relieving lower back pain.
In this particular case the iminothioether group -N=C-SCH3 linked to a six atom ring is the significant structural element which is shared by all the alternatives. Thus, since all the claimed compounds are alleged to possess the same use, unity would be present.
10.47 Example 27 (Common Structure)

wherein R1 is methyl or phenyl, X and Z are selected from oxygen (O) and sulfur (S).
The compounds are useful as pharmaceuticals and contain the 1,3-thiazolyl substituent which provides greater penetrability of mammalian tissue which makes the compounds useful as relievers for headaches and as topical anti-inflammatory agents.
All compounds share a common chemical structure, the thiazole ring and the six atom heterocyclic compound bound to an imino group, which occupy a large portion of their structure. Thus, since all the claimed compounds are alleged to possess the same use, unity would be present.
10.48 Example 28 (Common Structure)

All of the above copolymers have in common a thermal degradation resistance property, due to the reduced number of free COOH radicals by esterification with X of the end COOH radicals which cause thermal degradation.
The chemical structures of the alternatives are considered to be technically closely interrelated to one another. A grouping in one claim is therefore allowed.
Claim 1: Catalyst for vapor phase oxidation of hydrocarbons, which consists of (X) or (X+a).
In this example (X) oxidizes RCH3 into RCH2OH and (X+a) oxidizes RCH3 further into RCOOH.
Both catalysts share a common component and a common activity as oxidation catalyst for RCH3. With (X+a) the oxidation is more complete and goes until the carboxylic acid is formed but the activity still remains the same.
A Markush grouping is acceptable in this case.
10.50 Example 30 (Multiple Structurally and Functionally Related Polynucleotides)
Claim 1: An isolated polynucleotide selected from the group consisting of the nucleotide sequences SEQ ID NOs: 1-10.
(Some Authorities presume that a claimed biological molecule is in isolated form and therefore do not require the claim to explicitly include the term "isolated" as above.)
The facts are the same as Example 35 except that the claimed polynucleotides all share a significant structural element and their corresponding mRNAs are expressed only in the hepatocytes of patients with disease Y. The corresponding mRNAs are not expressed in the hepatocytes of healthy individuals.
There is no prior art available. The shared structural element had not been identified before, nor had any link been established between genes expressing mRNA containing that structural element and patients afflicted with disease Y.
The polynucleotides of claim 1 would be regarded as having the same or corresponding technical feature if the alternatives had a common property or activity, and shared a significant structural element that is essential to the common property or activity. Some Offices may regard claim 1 as a Markush grouping.
In this example, the description discloses that SEQ ID NOs: 1-10 share a common property, that is, expression of an mRNA present only in patients afflicted with disease Y. Moreover, SEQ ID NOs: 1-10 share a significant structural element that is essential to the common property, i.e., a probe comprising the shared structural element can detect the mRNA of patients afflicted with disease Y. Since both of these requirements are met, the group of polynucleotide molecules claimed meets the requirement of unity of invention (a priori).
No Unity of Invention (a priori) - Examples 31 to 39
Claim 1: Control circuit A for a d.c. motor.
Claim 2: Control circuit B for a d.c. motor.
Claim 3: An apparatus including a d.c. motor with control circuit A.
Claim 4: An apparatus including a d.c. motor with control circuit B.
Control circuit A is a special technical feature and control circuit B is another unrelated special technical feature.
Unity exists between claims 1 and 3 or between claims 2 and 4, but not between claims 1 and 2 or 3 and 4.
10.52 Example 32 (Common Structure)

The compound obtained by esterifying the end COOH radical of known polyhexamethyleneterephthalate with 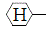 CH2O- has a thermal degradation resistant property, due to the reduced number of free COOH radicals which cause thermal degradation. In contrast, the compound obtained by esterifying the end COOH radical of known polyhexamethyleneterephthalate with a vinyl compound containing a CH2 = CH
CH2O- has a thermal degradation resistant property, due to the reduced number of free COOH radicals which cause thermal degradation. In contrast, the compound obtained by esterifying the end COOH radical of known polyhexamethyleneterephthalate with a vinyl compound containing a CH2 = CH  CH2O- moiety serves as a raw material for a setting resin when mixed with unsaturated monomer and cured (addition reaction).
CH2O- moiety serves as a raw material for a setting resin when mixed with unsaturated monomer and cured (addition reaction).
All esters covered by the claim do not have a property or activity in common. For example, the product obtained through esterification with the "CH2 = CH" vinyl compound does not have a thermal degradation resistant property. The grouping in a single application is not allowed.
10.53 Example 33 (No Common Structure)
Claim 1: A herbicidal composition consisting essentially of an effective amount of the mixture of A 2,4-D(2,4-dichlorophenoxy acetic acid) and B a second herbicide selected from the group consisting of copper sulfate, sodium chlorate, ammonium sulfamate, sodium trichloroacetate, dichloropropionic acid, 3-amino-2,5-dichlorobenzoic acid, diphenamid (an amide), ioxynil (nitrile), dinoseb (phenol), trifluralin (dinitroaniline), EPTC (thiocarbamate), and simazine (triazine) along with an inert carrier or diluent.
The different components under B must be members of a recognized class of compounds. Consequently in the present case a unity objection would be raised because the members of B are not recognized as a class of compounds, but, in fact, represent a plurality of classes which may be identified as follows:
(a) inorganic salts:
copper sulfat
sodium chlorate
ammonium sulfamate
(b) organic salts and carboxylic acids:
sodium trichloroacetate
dichloropropionic acid
3-amino-2,5-dichlorobenzoic acid
(c) amides:
diphenamid
(d) nitriles:
ioxynil
(e) phenols:
dinoseb
(f) amines:
trifluralin
(g) heterocyclic:
simazine
Claim 1: A pharmaceutical compound of the formula:
A - B - C - D - E
wherein:
A is selected from C1-C10 alkyl or alkenyl or cycloalkyl, substituted or unsubstituted aryl or C5-C7 heterocycle having 1-3 heteroatoms selected from O and N;
B is selected from C1-C6 alkyl or alkenyl or alkynyl, amino, sulfoxy, C3-C8 ether or thioether;
C is selected from C5-C8 saturated or unsaturated heterocycle having 1-4 heteroatoms selected from O, S or N or is a substituted or unsubstituted phenyl;
D is selected from B or a C4-C8 carboxylic acid ester or amide; and
E is selected from substituted or unsubstituted phenyl, naphthyl, indolyl, pyridyl, or oxazolyl.
From the above formula no significant structural element can be readily ascertained and thus no special technical feature can be determined. Lack of unity exists between all of the various combinations. When determining the first invention, one approach is to take into account the content of the dependent claims.
Another approach is for the first invention to be taken as the first mentioned structure for each variable, that is, A is C1 alkyl, B is C1 alkyl, C is a C5 saturated heterocycle having one O heteroatom, D is C1 alkyl, and E is a substituted phenyl. Dependent claims that are limited to this first invention may be considered as unified with the first invention and could all be searched without requiring an additional fee.
A further approach by some Authorities is to consider the first invention more broadly. For example, the first invention may be a compound wherein A is C1-C10 alkyl, B is C1-C6 alkyl, C is a C5-C8 saturated heterocycle having one O heteroatom, D is C1-C6 alkyl, and E is a substituted or unsubstituted phenyl. In such cases, further combinations may be identified for which additional search fees are invited. For example, a second election could be a compound wherein A is alkenyl, B is alkenyl, C is a substituted or unsubstituted phenyl, D is C4-C8 carboxylic acid ester, and E is a naphthyl. In such cases, it may also be appropriate to consider the description and the examples in order to identify specific groups of compounds for which to invite additional search fees.
The grouping of claims containing many variables should be determined on a case by case basis consistent with the principles previously set out in these guidelines.
10.55 Example 35 (Multiple Structurally and Functionally Unrelated Polynucleotides)
Claim 1: An isolated polynucleotide selected from the group consisting of the nucleotide sequences SEQ ID NOs: 1-10.
(Some Authorities presume that a claimed biological molecule is in isolated form and therefore do not require the claim to explicitly include the term "isolated" as above.)
The description discloses that the claimed polynucleotides are 500 bp cDNAs obtained from a human liver cDNA library. The polynucleotides are structurally different and can be used as probes to obtain full-length DNAs, although there is no description of the function or biological activity of the corresponding proteins. Furthermore, the polynucleotides claimed are not homologous to each other.
There is no prior art available. A human liver cDNA library had not been established before.
The polynucleotides of claim 1 would be regarded as having the same or corresponding technical feature if the alternatives had a common property or activity, and shared a significant structural element that is essential to the common property or activity, as determined by the Authority (see paragraph 10.05). Some Offices may regard claim 1 as a Markush grouping.
In this example, the description fails to disclose that all of the polynucleotides SEQ ID NOs: 1-10 share a common property or activity. While each sequence may serve as a probe to isolate its own respective full length DNA, due to the lack of homology between SEQ ID NOs: 1-10, a probe derived from SEQ ID NO: 1 cannot be used to isolate SEQ ID NOs: 2-10, respectively.
Moreover, since the polynucleotides are not homologous to each other, they fail to share a common structure i.e., a significant structural element. The sugar-phosphate backbone cannot be considered a significant structural element, since it is shared by all nucleic acid molecules. Therefore, the 10 polynucleotide molecules do not share any significant structural element and cannot be considered as having the same or corresponding technical feature.
The mere fact that polynucleotide fragments are derived from the same source (human liver) is not sufficient to meet the criteria for unity of invention. The polynucleotides fail to share a common property or activity and fail to share a common structure, as determined by the Authority. Since neither of these two requirements is met, the group of polynucleotide molecules claimed does not meet the requirement of unity of invention (a priori).
One possible grouping would be:
Inventions 1-10: Polynucleotides having SEQ ID NOs: 1-10.
10.56 Example 36 (Functionally Unrelated Single Nucleotide Polymorphisms (SNPs))
Claim 1: An isolated nucleic acid molecule comprising SEQ ID NO: 1 with a single polymorphic change at one of the positions as shown below:
Polymorphism Position Change from SEQ ID NO: 1 to:
1 10 G
2 27 A
3 157 C
4 234 T
5 1528 G
6 3498 C
7 13524 T
8 14692 A
(Some Authorities presume that a claimed biological molecule is in isolated form and therefore do not require the claim to explicitly include the term "isolated" as above.)
According to the description, SEQ ID NO: 1 is 22,930 nucleotides in length. The SNPs 1-8 are not characterized, that is, no common property or activity has been disclosed.
SEQ ID NO: 1 has been described in the prior art but no specific function has been identified.
The polynucleotides of claim 1 would be regarded as having the same or corresponding technical feature if the alternatives had a common property or activity, and shared a significant structural element that is essential to the common property or activity. Some Offices may regard claim 1 as a Markush grouping.
In this example, the description fails to disclose that all of the SNPs 1-8 share a common property or activity. The fact that all point mutations are within a defined sequence (SEQ ID NO: 1) is not sufficient to establish unity of invention since SEQ ID NO: 1 has already been described in the prior art, and no functional relationship exists among the different SNPs claimed. For this reason, the SNPs of claim 1 lack unity of invention.
One possible grouping would be:
Inventions 1-8: SNPs 1-8.
10.57 Example 37(Molecules Which Share a Common Function not Linked to a Common Structure)
Claim 1: A fusion protein comprising carrier protein X linked to a polypeptide having SEQ ID NO 1, 2, or 3.
The description discloses that carrier protein X is 1000 amino acids in length and functions to increase the stability of the fusion proteins in the blood stream. SEQ ID NOs: 1, 2, and 3 are small epitopes (10-20 residues in length) isolated from different antigenic regions of E.coli. SEQ ID NOs: 1, 2, and 3 do not share any significant common structure.
Both the structure of protein X and its function as a carrier protein are known in the prior art. Fusion proteins that generate an antigenic response to E. coli are known in the prior art.
The fusion proteins of claim 1 would be regarded as having the same or corresponding technical feature if the alternatives had a common property or activity, and shared a significant structural element that is essential to the common property or activity, as determined by the Authority (see paragraph 10.05). Some Offices may regard claim 1 as a Markush grouping.
In this example, the only common structure shared by the fusion proteins is carrier protein X. The fusion proteins share a common property, i.e., generation of an antibody response specific for E. coli. However, immunization with the carrier protein alone does not result in the common property; SEQ ID NO: 1, 2, or 3 is required for this property.
No special technical feature exists among the three fusion proteins. The fact that all the fusion proteins have a common property is not sufficient to establish unity of invention because (1) SEQ ID NOs: 1, 2, and 3, which impart the common property, do not share a significant structural element, (2) the common structure, carrier protein X, does not impart the common property, and (3) fusion proteins that generate an antigenic response specific for E. coli are known in the prior art.
One possible grouping would be:
Invention 1: Fusion protein comprising carrier protein X and SEQ ID NO: 1.
Invention 2: Fusion protein comprising carrier protein X and SEQ ID NO: 2.
Invention 3: Fusion protein comprising carrier protein X and SEQ ID NO: 3.
10.58 Example 38 38 (Multiple Nucleic Acid Molecules Which Share Common Structure and Encode Proteins with Common Property)
Claim 1: An isolated nucleic acid selected from SEQ ID NO: 1, 2, or 3.
(Some Authorities presume that a claimed biological molecule is in isolated form and therefore do not require the claim to explicitly include the term "isolated" as above.)
The description discloses that the three nucleic acids encode dehydrogenases that include a conserved sequence motif defining the catalytic site and the dehydrogenase function of these proteins. The three nucleic acids were isolated from three different sources (mouse, rat, and human). The description clearly shows that these three nucleic acids are homologous based upon their overall sequence similarity (85-95% identity) at both the nucleotide and amino acid sequence levels.
The prior art describes a nucleic acid molecule isolated from monkeys, which has high sequence similarity (e.g., 90%) to SEQ ID NO: 1. The monkey nucleic acid encodes a dehydrogenase that includes the catalytic site defined by the conserved motif.
The nucleic acids of claim 1 would be regarded as having the same or corresponding technical feature if the alternatives had a common property or activity, and shared a significant structural element that is essential to the common property or activity. Some Offices may regard claim 1 as a Markush grouping.
Rule 13.2 requires that the technical feature shared between the inventions defines a contribution over the prior art.
A same or corresponding technical feature shared among the claimed nucleic acid molecules resides in their common property (encoding dehydrogenases) and their shared structural element that is essential to the common property (the conserved motif). However, a nucleic acid molecule which encodes a dehydrogenase and contains the shared structural element has already been isolated from a different source (monkeys). Thus, the technical feature is not special because the functional and structural similarity between the claimed molecules cannot form the contribution that the group of inventions as a whole makes over the prior art. Therefore, unity of invention is lacking (a posteriori).
On the other hand, if the only prior art available disclosed a nucleic acid molecule encoding a dehydrogenase that lacked the catalytic site defined by the conserved sequence motif, the technical feature would be special and SEQ ID NOs: 1, 2, and 3 would have unity of invention.
A possible grouping would be:
Invention 1: Nucleic acid of SEQ ID NO: 1
Invention 2: Nucleic acid of SEQ ID NO: 2
Invention 3: Nucleic acid of SEQ ID NO: 3
10.59 Example 39 (DNA Encoding Receptors with Partial Structural Identity and Asserted Common Property)
Claim 1: A polynucleotide encoding a guanosine triphosphate-binding protein coupled receptor (GPCR) comprising a nucleotide sequence selected from the group consisting of the odd-numbered SEQ ID NOs from SEQ ID NO: 1 to SEQ ID NO: 2069.
The description identifies a conserved sequence of 15 amino acid residues found in several known GPCR molecules that is asserted to be essential to the GPCR function. A consensus polynucleotide sequence encoding the conserved amino acid sequence was generated. A database containing human genome sequences was searched using the consensus polynucleotide sequence. Using this system, 1035 polynucleotide sequences were identified, which are asserted to encode GPCR molecules that include the conserved sequence.
The prior art discloses human GPCR molecules that contain the conserved sequence of 15 amino acid residues, as well as the polynucleotide sequences that encode the conserved 15 amino acid sequence.
The common technical feature among the 1035 polynucleotide sequences is the consensus polynucleotide sequence that encodes the common sequence of 15 amino acid residues. This technical feature is not special because the consensus polynucleotide sequence was known and therefore cannot form the contribution that the group of inventions as a whole makes over the prior art. Consequently, the 1035 different polynucleotides lack unity of invention (a posteriori).
One possible grouping would be:
Inventions 1-1035: Polynucleotides based on SEQ ID NOs: 1-2069 (odd-numbers)
If the description did not assert, or it was not readily apparent, that the conserved sequence of 15 amino acid residues was essential to the GPCR function, unity of invention could be lacking in the absence of any relevant prior art.
On the other hand, given the assertion in the description, in the absence of the prior art in the example, the groups would have had unity of invention.
Dependent Claims Adding Substantial Features Which Diverge from the Inventive Concept (Lack of Unity a posteriori) - Example 40
Claim 1: A humidifier, comprising:
a tub to contain a supply of water; an inlet to receive a flow of breathable gas, the inlet configured to direct the flow over the supply of water to humidify the flow;
an outlet connectable to a conduit; a wicking element provided in the tub; and
a heating element extending from the inlet to the outlet, wherein the heating element is configured to contact the supply of water.
Claim 2: A humidifier according to claim 1, wherein the heating element comprises:
at least one resistive wire having a first end and a second end;
an insulating layer between the first and second ends; and
an outer coating surrounding the at least one resistive wire and the insulating layer.
Claim 3: A humidifier according to claim 1, further comprising a support in the tub to support the wicking element, wherein the support is a tubular support and the wicking element is provided on an outer surface of the tubular support.
In this example the features of claim 1 are found to be disclosed in the prior art and are hence not novel and not inventive. In addition, claims 2 and 3 define substantially different special technical features and are further directed to substantially different technical aspects. Claims 2 and 3 lack unity a posteriori, as long as this consideration is consistent with paragraph 10.04 which states that "If the common matter of the independent claims is well known and the remaining subject matter of each claim differs from that of the others without there being any unifying novel inventive concept common to all, then clearly there is lack of unity of invention. If, on the other hand, there is a single general inventive concept that appears novel and involves inventive step, then objection of lack of unity does not arise. For determining the action to be taken by the examiner between these two extremes, rigid rules cannot be given and each case is considered on its merits, the benefit of any doubt being given to the applicant".
Lack of Unity of Invention in a Single Independent Claim - Examples 41 and 42
Claim 1: A method of detecting bladder cancer in a subject comprising:
(a) contacting a sample obtained from the subject with one or more agents that detect expression of at least one of the markers chosen from MAGEA 10, DSCR8, MMP 12, CXCL9, DSCR8, KRT81, LOC729826, PTHLH, MMP1 1, and S100A7; and;
(b) contacting a non-cancerous cell, e.g. a non-cancerous cell from bladder tissue or a non-cancerous bladder cell line, with the one or more agents that detect expression of at least one of the markers listed above;
wherein a higher level of expression of one or more of the markers in the sample compared to the non-cancerous cell indicates that the subject has bladder cancer.
According to "Markush practice", a claim defining alternatives can be unified when the alternatives share a common property or activity and either have a common structure or belong to a recognized class of compounds.
A "recognized class of compounds" must be a class of compounds already known based on the prior art (e.g., TNF inhibitors, tumor suppressors, serine threonine kinases) that a skilled addressee would expect will behave in the same way.
In the present claim, while the alternatives have a common property, namely their role as a biomarker for bladder cancer, the alternatives do not have a common structure; and are not considered to be a recognized class of chemical compounds because each of the biomarkers identified come from diverse gene/protein families. Therefore, each biomarker is considered to be a separate invention.
Also note that the association between bladder cancer and biomarkers has been disclosed in the prior art and cannot itself represent a special technical feature.
Claim 1: A method of forming an orthotic for a patient's foot, the method comprising the steps of:
preparing an orthotic template for the foot wherein the template extends between a heel end and a toe end, preparing the template including the steps of:
attaching an upper layer of thermoplastic material to a lower layer of thermoplastic material or heating the prepared orthotic template to a predetermined temperature.
The claim can be written into two different independent claims (a) or (b).
(a) A method of forming an orthotic for a patient's foot, the method comprising the steps of:
preparing an orthotic template for the foot wherein the template extends between a heel end and a toe end, preparing the template including the steps of:
attaching an upper layer of thermoplastic material to a lower layer of thermoplastic material.
or
(b) A method of forming an orthotic for a patient's foot, the method comprising the steps of:
preparing an orthotic template for the foot wherein the template extends between a heel end and a toe end, preparing the template including the steps of:
heating the prepared orthotic template to a predetermined temperature.
The feature of "forming an orthotic for a patient's foot" by "preparing an orthotic template for the foot wherein the template extends between a heel end and a toe end" is common to claims (a) and (b).
However, if it can be established that this common feature is known in the art, then there will be a lack of unity of invention a posteriori within the single independent claim.
Complex Claim Sets with Overlapping Features - Example 43
Often claims contain features that overlap with the features of other claims. Determination of unity of invention in these cases requires careful consideration. The lack of unity observation will depend on the facts of the case, and care should be taken that an objection is not raised on the basis of a narrow, literal or academic approach, as warned against in paragraph 10.04.
Claim 1: A turbine rotor blade formed to provide a semicircular shaped cross section.
Claim 2: A turbine rotor blade as claimed in claim 1 comprising alloy Z.
Claim 3: Alloy Z.
Independent claim 1 is directed to a turbine blade. The feature of 'the blade formed to provide a semicircular shaped cross section' is considered to be the special technical feature of this claim.
Independent claim 3 is directed to an "alloy Z" and this is considered to be the special technical feature of this claim.
Therefore, independent claims 1 and 3 lack unity a priori as there is no special technical feature common to these claims.
If claim 1 is novel and inventive, then, according to paragraph 10.07, it follows that there is unity of invention in respect of any claims that depend on the novel claim 1. That is, unity of invention exists between claims 1 and 2.
If after a review of the prior art claim 1 is found to be not novel or not inventive; that is if "a turbine rotor blade formed to provide a semicircular shaped cross section" is known in the art, and alloy Z is found to be novel and inventive, it follows that there is unity of invention between claims 2 and 3, as both contain a common special technical feature, namely alloy Z.
However, if alloy Z is not novel or inventive, then any lack of unity of invention between claims 2 and 3 would be a purely academic exercise.
In all of the above scenarios, independent claims 1 and 3 lack unity a priori as there is no special technical feature common to these claims. However, the appropriate grouping of the claims will depend on the facts of the case.