Where Science Meets Sleep: Technology Transfer Journey of the University of Cape Town’s Innovation for Obstructive Sleep Apnea to Address a Global Health Challenge.
Obstructive Sleep Apnea (OSA) is a pressing global health issue, affecting nearly one billion people worldwide. It happens when the soft parts of the throat relax too much during sleep and block the airway. This makes it hard to breathe and causes repeated pauses in breathing throughout the night. These interruptions can lead to other health issues like heart disease, high blood pressure, stroke, problems with metabolism, feeling very tired during the day, and even early death.

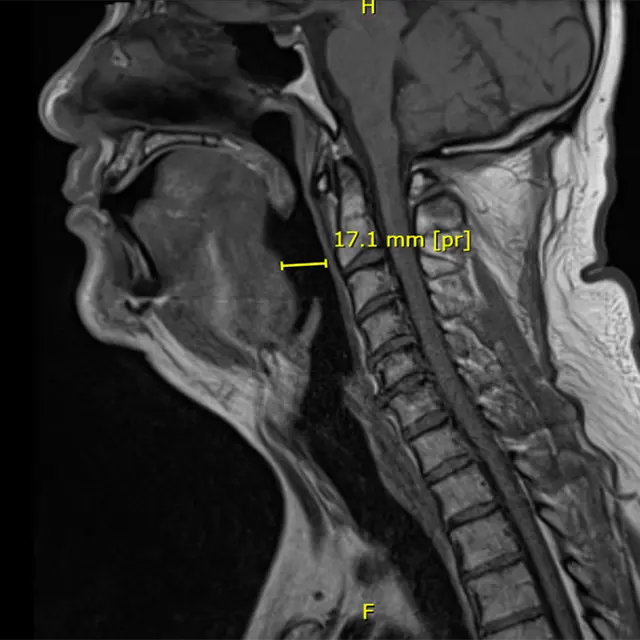
Device in-situ (Image: Francois Oosthuizen)
Although there are different treatments available, OSA still affects a huge number of people, showing that new and better solutions are needed. At the University of Cape Town (UCT), a collaboration between Professor Rushdi Hendricks, a maxillofacial surgeon, and the late Professor Deon Bezuidenhout, a polymer science specialist, sparked a groundbreaking idea: what if the body could grow its own support structure to keep the airway open?
Their answer was an innovative implant called the Biological Tendon-like Tether Technology (BTTT). It is a small scaffold that helps the body grow its own tissue to gently hold the airway open during sleep. The scaffold is placed at the base of the tongue, where it helps the body grow strong collagen fibers. These fibers eventually replace the implant and do the job naturally.
The invention is inducing the body to grow a tendon-like tether… It’s your own body, it’s your own anatomy. You’ve grown your own cure.
Francois Oosthuizen, Innovation Commercialization Manager, UCT Research Contracts and Innovation (RC&I)
But developing this tissue-engineering technology was just the beginning. Turning it into a real-world solution required navigating the complex route of technology transfer.
Turning OSA Implant Idea into a Real-World Medical Solution
Turning this innovative obstructive sleep apnea implant device into something that could help real patients didn’t happen overnight. It took a lot of planning, support, and teamwork. At UCT, the Research Contracts and Innovation (RC&I) office helped guide the invention through every stage of the commercialization pipeline.

The process started in 2014, when the inventors disclosed their concept to RC&I. The team looked carefully at whether the idea could be protected through patents, whether it could realistically be built and used, how many people it could help, and how easily it could be produced. The intellectual property (IP) potential was assessed by Mr. Philip Hoekstra, IP Manager at RC&I.
Recognizing its potential, RC&I secured pre-seed funding from South Africa’s Technology Innovation Agency (TIA), which allowed the researchers to test the idea in pre-clinical studies using animal models. These tests, conducted between 2015 and 2016, were successful and provided the basis for the filing of a provisional patent application in the United Kingdom in 2016. This was followed in 2017 by an international patent application through the Patent Cooperation Treaty (PCT), administered by WIPO.

The PCT route enabled the team to secure patent protection in key global markets, including the United Kingdom, European Union, United States, China, and South Africa. These regions have significant medical device markets and high OSA prevalence.
Additional support came from the National Intellectual Property Management Office (NIPMO), which helped cover patent costs, and funded the creation of materials, such as marketing flyers, brochures and digital content created by RC&I, to explain the technology to potential partners and investors.

You don’t just walk in and pick up a patent to commercialize. It’s years of work, building relationships with researchers, understanding the commercial context, refining prototypes, showing data, and earning industry trust.
Francois Oosthuizen
One of the biggest challenges in developing a new medical device is convincing others, especially investors, that it’s safe, effective, and worth backing. That’s why this early phase focused on de-risking. With RC&I’s support, the inventors took important steps to make the project more attractive to future funders, moving it from a bold idea to a believable, practical solution.
Navigating Patent Strategy for the Implant for Obstructive Sleep Apnea
Securing a robust intellectual property (IP) position was foundational. In addition to filing patent applications in key markets, RC&I undertook a “freedom-to-operate (FTO)” analysis. This step is essential to ensure that the BTTT technology would not infringe anyone else’s existing IP rights. While the scaffold design itself was unique and free of conflict, the team identified that one of the original production techniques could potentially conflict with an existing patent.
Fortunately, that third-party patent had lapsed, resolving the concern. However, another challenge emerged: the inventors initially had access to the base polymer material only for research purposes. To move forward, RC&I successfully negotiated a commercial-use license from the material’s rights holder, ensuring full FTO to produce and use the technology in treating OSA.
Throughout the process, RC&I worked closely with experienced South African patent attorneys to craft a strong international patent strategy. They also used resources from WIPO, such as the PATENTSCOPE database, to effectively assess global patent landscapes, structure filings and to assist with the FTO assessment.
Prototyping of New Sleep Apnea Treatment: BTTT
Before human testing, extensive prototyping was needed. Initial versions were tested for cardiovascular use, yielding valuable insights. The team then developed refined BTTT OSA implant prototypes using proprietary manufacturing methods and tested them in sheep, confirming biocompatibility and structural strength.
The next milestone: human clinical validation. This required building an ISO13485-compliant manufacturing process, developing a clinical protocol in line with ISO14155, navigating South Africa’s regulatory system, and securing investment for trials.
In 2024, the South African Health Products Regulatory Authority (SAHPRA) approved the first human trials. Conducted with UCT’s Clinical Research Centre, the early results showed strong promise for treating OSA.
Medical experts and quality assurance partners contributed throughout the design process, ensuring surgical practicality and regulatory readiness. This collaborative, iterative work showcases a successful technology transfer.
Taking New OSA Treatment from Lab to Market
Valuing a novel medical device at an early stage before revenue or large-scale trials is notoriously difficult. With no revenue yet generated and significant clinical work still ahead, BTTT faced what is commonly referred to as the “valley of death” in translational medicine, or a stage where promising innovations struggle to secure the funding needed to move forward. Traditional investors were hesitant, especially in the absence of human clinical data.
UCT’s Innovation Builder Fund, a high-risk grant facility, provided crucial early-stage support. This sustained momentum while the team sought external investors. A key development partner was Life Healthcare, a leading South African hospital group. Their support for clinical trials and access to top-tier facilities proved instrumental in progressing BTTT from lab to clinic.
As interest grew, especially from the Middle East, a more advanced commercialization plan took shape. Now, Professor Hendricks and his commercial team are working with RC&I to create an appropriate investment structure that can fully unlock the technology's value. The initial strategy would be to license the OSA technology and eventually assign the IP asset to an external investment or commercial vehicle at a later stage. In exchange, UCT would retain a stake, either through royalties, equity, or a combination of both.
A core principle: any transfer of IP would be at market value, ensuring fair returns for UCT, inventors, and society.
How to Treat OSA using the BTTT Implant - the Value of Know-How
Beyond patents, a large part of this project’s value lies in the specialized know-how developed by the team. This includes the production process of the tissue scaffold, the surgical protocols, patient care pathways, and the quality management systems, refined through years of trial, collaboration, and hands-on learning.

To make sure this know-how is preserved and can be shared with others, the team has carefully codified all key processes: from surgical training curricula, cadaver labs to digital modules for surgeon certification. The clinical protocol itself has been formalized and now serves as a blueprint for replication in new settings.
This structured, codified expertise will become essential as the technology scales internationally. It will help ensure consistent treatment outcomes, enable effective training, and increase investor confidence by reducing dependency on tacit, person-specific knowledge that is difficult to transfer.
Facilitating Use of BTTT Treatment for Sleep Apnea
The BTTT team is now focused on expanding both production capabilities and surgeon training frameworks. Efforts include potentially transitioning to an alternative production method that would support high-volume, low-cost manufacturing while enhancing quality control. Simultaneously, efforts are underway to refine the surgical technique, aiming to make the procedure less invasive, reducing both the treatment's time and costs.
To support global expansion, the team is designing training programs that can be implemented internationally, with particular attention in regions such as the United States, European Union, and China, where the prevalence and economic impact of OSA are specifically high.
The broader vision is not only to build capacity to distribute devices but also to empower healthcare systems through knowledge transfer and local capacity-building, ensuring that the innovation can be adopted, sustained, and scaled for long-term impact.
Redefining Regenerative Medicine for Treating Obstructive Sleep Apnea

We, as tech transfer professionals, work in the industry of hope! Every day, we collaborate with people who bring us ideas that can change the world. This never becomes routine. Every day, I get to see hopes and dreams realized. And I get to play a part in that.
Francois Oosthuizen
The journey of BTTT embodies that spirit. It is not just a story of scientific achievement or patents. It is a story about people. About visionary thinkers who saw a problem affecting millions and dared to imagine a better solution. About the teams who worked behind the scenes to translate that idea into something real, impactful, and enduring.
Through careful strategy, continuous de-risking, and a deep commitment to excellence, this South African-born innovation now stands ready to reshape how the world treats OSA and perhaps redefine regenerative medicin.
While commercialization is still in its early stages, BTTT shows what is possible when clinical insight, material science, and dedicated tech transfer align. From a university laboratory in Cape Town to the global health stage, this is more than a story about technology.
It is a story of hope – grown, nurtured, and ready to change lives.

The Role of WIPO in Supporting the BTTT Journey
In 2023, WIPO deepened its collaboration with UCT by sending a staff member, Lien Verbauwhede Koglin, to the Research Contracts and Innovation (RC&I) office. This exchange provided WIPO with direct insight into the realities of technology transfer in South Africa and allowed UCT to engage more closely with global best practices.
In addition, UCT’s IP policy approach was shaped by South Africa’s IPR Act and NIPMO’s Guideline 8.1 of 2021, which is based on WIPO’s model IP policy templates. This helped the university create a solid framework for responsible IP management and sustainable commercialization. UCT also used WIPO’s Incentives Guide for Researchers and Technology Transfer Professionals and IP Policies Database to design fair benefit-sharing and reward structures essential for spin-off planning.
The collaboration demonstrated how WIPO’s tools and resources can effectively support innovation ecosystems in Member States.