
WIPO’s Budapest Treaty facilitates biotech patenting
Humans have been using microorganisms for millennia. Tiny, single-cell living organisms such as yeast and bacteria are essential to produce food products like wine, beer and cheese. Only in the 20th century, however, did the industrial application of these microscopic powerhouses take off. Greater understanding of biological processes, thanks in large part to Watson and Crick’s work on DNA, paved the way for the development of revolutionary techniques such as genetic engineering, enabling scientists to manipulate microorganisms in spectacular new ways, to enormous social benefit.
In the medical field, microorganisms are used to produce a host of life-saving therapies – antibiotics, vaccines, insulin – and diagnostic tools; in agriculture, they are used in developing high-yielding, resistant crop varieties. They are also used in environmental waste management systems and a host of industrial applications, including the production of green fuels like ethanol. These tiny organisms have huge potential to improve the quality of our lives and the environment in which we live, and to reduce our carbon footprint.
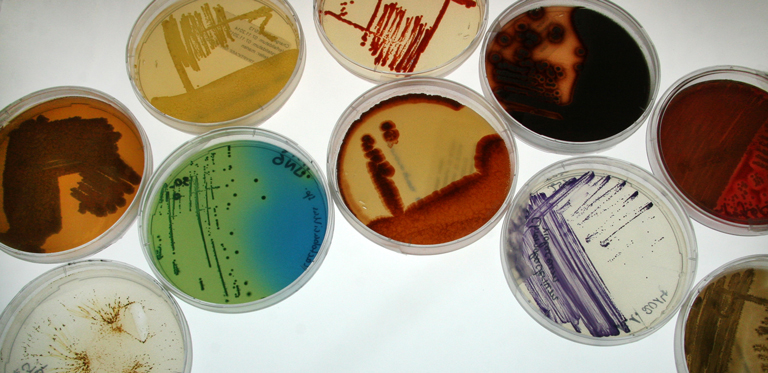
For many, biotechnology holds the key to overcoming some of the daunting challenges facing humanity in the 21st century.
Developing these groundbreaking applications takes a massive investment of time, energy and resources. It is a high-risk research undertaking, and successful innovations can be imitated at little cost. As such, researchers and the biotech companies that employ them rely heavily on the intellectual property system, especially patents, to protect their know-how and maximize the chances of getting a return on their investment.
Criteria for patent protection
Applicants seeking patent protection in all fields of technology are required to satisfy certain criteria as set out in national patent law. Typically, to qualify for patent protection an invention must be novel, non-obvious to a specialist working in the relevant field and must have some industrial application or utility. Within the patent application process, there is what is known as a disclosure requirement whereby applicants must describe how their invention works. The description must be sufficiently detailed for a specialist in the field to be able to put the invention into practice – the enablement requirement.
These requirements are an important part of the social bargain that underpins the patenting process. An applicant receives the protection conferred by a patent and in return publicly discloses details of his or her invention so that others may improve upon it and develop a better technology, and thereby push the frontiers of technological development. This patent-related information is stored in powerful databases such as WIPO’s PATENTSCOPE, the world’s largest publicly available patent database which currently hosts over 47 million patent applications, and use of which is free of charge.
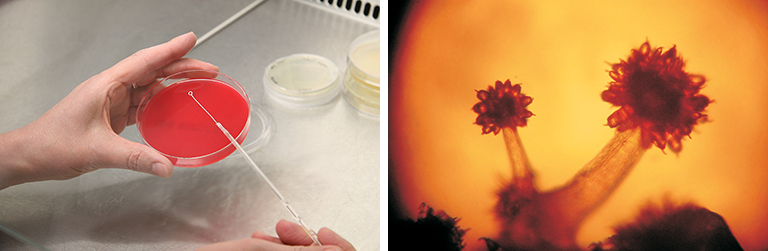
How biotech patenting is different
For many technologies, a written description is enough to enable a specialist working in the relevant field to reproduce an invention for which patent protection is sought. When it comes to microorganisms, however, this will not generally suffice. Take, for example, an organism isolated in soil that has been “improved” by mutation and further selection. It would be practically impossible to describe the strain and its selection in a way that would guarantee that another skilled microbiologist would obtain the same strain. In such instances, the microorganism itself is considered a key part of the disclosure ![]() . For this reason, many countries require that when patenting microorganisms, written disclosure is complemented by deposit of the biological material in question with a specialized culture collection.
. For this reason, many countries require that when patenting microorganisms, written disclosure is complemented by deposit of the biological material in question with a specialized culture collection.
However, depositing multiple samples with each patent application is impractical. IP offices are ill-equipped to store and preserve biological materials and such a requirement would be hugely time-consuming and costly.
An international mechanism that facilitates biotech patenting
Recognizing the peculiar challenges of patenting microorganisms, and the need for a streamlined and cost-effective international procedure, in the late 1970s policymakers adopted the WIPO-administered Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure.
A key advantage of the Budapest Treaty is that for the purposes of patenting procedures, it eliminates the need to deposit multiple samples of the same biological material with biological resource centers in different countries. As such, it offers applicants an efficient, streamlined and cost-effective means of meeting the disclosure requirements associated with patenting microorganisms and other biological material.
Accession to the Budapest Treaty by countries or competent intergovernmental organizations does not require any substantive change to their national or regional patent legislation because the Treaty itself does not define a microorganism nor does it regulate any patentability requirements.
The main users and participants in the Budapest Treaty System are patent offices, depositors of biological material, patent applicants, patent lawyers, scientists and international depositary authorities (IDAs).
A key role for national culture collections
The Treaty recognizes certain biological resources centers or culture collections as IDAs where patent-related samples of biological material can be deposited and stored (thereby also fulfilling the need for disclosure information to be made publicly available). There are currently 45 IDAs in operation around the world, and biological material deposited with any one of them is recognized by all members of the Treaty as “valid for patent purposes by all countries in which protection for the relevant invention is sought.” To date, 79 countries have signed up to the Budapest Treaty.
Any biological resource center or culture collection can become an IDA under the Budapest Treaty if it meets certain conditions and is formally nominated by a member country. These institutions specialize in the collection and storage of specific types of biological material which they make available for research purposes. For example, the German Collection of Microorganisms and Cell Cultures (Leibniz Institut - Deutsche Sammlung von Mikrooganismen und Zellkulturen GmbH, (DSMZ)) hosts an open collection of over 35,000 cultures of archaea, bacteria, genomic DNAs, bacteriophages, fungi, yeasts, plant cell cultures, plant viruses and animal and human cell cultures which it makes available to scientists around the world.
All IDAs comply with certain requirements; in particular, they agree to accept and store deposited materials for at least 30 years or five years after the most recent request for a sample, whichever is later. They also agree to provide samples of deposited material only to those entitled to receive them (e.g. anyone with the depositor’s written authorization or an “interested” patent office). Storing bio materials and processing samples for patenting procedures still entails costs but these are significantly reduced thanks to the Budapest Treaty.
DSMZ began operating as an IDA under the Budapest Treaty in 1981. As such, it serves as “a center for the safe deposit of biological material for patent purposes,” notes Dr. Vera Bussas, DSMZ’s IDA Representative responsible for managing its patent deposit collection. With more than 8,000 deposits filed under the Budapest Treaty, and a capacity to accept a broad range of biological materials, DSMZ is one of the largest IDAs in the world.

Upon receipt of a patent-related sample, DSMZ checks the viability and purity of the biological material deposited. This can take several days or weeks, depending on the type of material and species of organism. It then issues a deposit receipt and a statement of viability (Forms BP/4 and BP/9). Such information typically has to be included in the patent application at the time of filing so some forward planning is required.
Making biological material available for research
“We then preserve and store the biological material for at least 30 years as prescribed by the Budapest Treaty,” Dr. Bussas explains. “Whenever possible, two methods of preservation, such as freeze-drying or storage in liquid nitrogen, are applied, and the viability of the cultures is inspected periodically,” she says.
“The main reason for depositing patent-related biological material with an IDA is to render it available to entitled parties for trials and examinations,” Dr. Bussas explains, noting that industry players deposit significantly more samples than their counterparts in research institutions. Every year some 2,000 samples are furnished by IDAs around the world. “DSMZ releases around 150 samples per year, mostly to industrial customers from abroad.”
Since the Budapest Treaty became operational in early 1981, over 90,000 patent-related samples of biological material have been deposited in IDAs around the world. In 2014, China (51 percent) and the United States (21.9 percent) accounted for 72.9 percent of deposits made. “The total number of deposits each year is still increasing, especially among Asian IDAs, which are showing amazing increases in deposition numbers,” Dr. Bussas notes.
Enabling biotech companies to capture value
“Recognizing the huge potential that biotechnology holds in treating human disease, many biopharmaceutical companies have made it a priority to discover and develop microorganisms to treat a wide range of medical conditions, including cancer, allergies and autoimmune and inflammatory diseases,” explains Emil Pot, Legal Counsel at ActoGeniX, a small biotech company based in Belgium.
“In the next decade, we will see even more investment in this important field and many more of these microbiome products will make their way to the market. By depositing these valuable biological materials via the Budapest Treaty, companies are able to pursue patent protection and thereby capture their commercial value, safeguard their rights and generate opportunities to fund further research,” Mr. Pot observes.
Biotech patents on the rise
With growing demand for biotechnology-related patents – the sector experienced a 4.7 percent growth rate in patents between 2007 and 2011 – the number of IDAs continues to increase. In 1990 there were just 10 IDAs, in 2000 there were 33 of them, and today 45 are in operation. The majority of IDAs – 27 of them – are located in Europe with four in North America, 10 in Asia, two in Australia and two in Latin America. At present, culture collections with IDA status are located in just four developing countries. Dr. Bussas is confident that this will change in the future: “As biotechnology starts budding in Africa and South America we will see the establishment of more IDAs in these areas.”
Culture collections with IDA status in developing countries
China
- The China Center for Type Culture Collection (CCTCC)
- The China General Microbiological Culture Collection Center (CGMCC)
Chile
- The Chilean Collection of Microbiotic Genetic Resources (CChRGM)
India
- The Microbial Culture Collection (MCC)
- The Microbial Type Culture Collection and Gene Bank (MTCC)
Mexico
- The Microorganism Collection of the National Center of Genetic Resources (CM-CNRG) (IDA status acquired in August 2015).
While anticipating the growth of the global IDA network, Dr. Bussas offers a word of caution. “A well-functioning culture collection must first be in place before institutions try to obtain IDA status,” she explains. “Countries active in the biotechnology sector need to think about joining the Budapest Treaty – only then can they benefit from its advantages in terms of uniform and cost-effective procedures.”
As biotechnological research continues to push the boundaries of possibility, and the number of biotech-related patents rises, the future looks bright for the Budapest Treaty and its expanding network of IDAs, not to mention the many biotech companies that save time and money by using them.
The WIPO Magazine is intended to help broaden public understanding of intellectual property and of WIPO’s work, and is not an official document of WIPO. The designations employed and the presentation of material throughout this publication do not imply the expression of any opinion whatsoever on the part of WIPO concerning the legal status of any country, territory or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication is not intended to reflect the views of the Member States or the WIPO Secretariat. The mention of specific companies or products of manufacturers does not imply that they are endorsed or recommended by WIPO in preference to others of a similar nature that are not mentioned.